Science, Systems, Matter, and Energy
... Feedback loop – when output of matter, energy, or information is fed back into the system as an input that changes the system. • Positive Feedback – causes change in the same direction • Negative Feedback – one change leads to a lessoning of that change. ...
... Feedback loop – when output of matter, energy, or information is fed back into the system as an input that changes the system. • Positive Feedback – causes change in the same direction • Negative Feedback – one change leads to a lessoning of that change. ...
Science Starter Tuesday Week 2
... If we can’t see air, how do we know it exists? List at least 3 examples of EVIDENCE or things you could do that helps us know that air exists. ...
... If we can’t see air, how do we know it exists? List at least 3 examples of EVIDENCE or things you could do that helps us know that air exists. ...
Nuclear Chemistry
... An isotope has the same atomic number but different mass number Isotopic notation Mass # ...
... An isotope has the same atomic number but different mass number Isotopic notation Mass # ...
Molecular Luminescence Spectroscopy
... Filters and Monochromators: Both interface and absorption filters have been used in fluorometers for wavelength selection of both the excitation beam and the resulting fluorescence radiation. Most spectrofluorometers are equipped with at least one and sometimes two grating monochromators. Transducer ...
... Filters and Monochromators: Both interface and absorption filters have been used in fluorometers for wavelength selection of both the excitation beam and the resulting fluorescence radiation. Most spectrofluorometers are equipped with at least one and sometimes two grating monochromators. Transducer ...
Modern Physics
... Dual Nature of Light Light exhibits wave phenomena as a light wave is propagated by interchange of energy between varying electric and magnetic fields (Maxwell) Light acts like particles composed of kinetic energy and momentum when light interacts with matter Both wave and particle 1. Wave Nat ...
... Dual Nature of Light Light exhibits wave phenomena as a light wave is propagated by interchange of energy between varying electric and magnetic fields (Maxwell) Light acts like particles composed of kinetic energy and momentum when light interacts with matter Both wave and particle 1. Wave Nat ...
41 Chapter 4 Atomic Structure 4.1 The Nuclear Atom J. J. Thomson
... Dynamically stable orbits, like those of the planets around the sun, would allow electrons to remain "attached" to nuclei. So let's calculate such an orbit. As you learned in Physics 23, there must be a centripetal force holding anything in circular motion (remember, circular motion is accelerated m ...
... Dynamically stable orbits, like those of the planets around the sun, would allow electrons to remain "attached" to nuclei. So let's calculate such an orbit. As you learned in Physics 23, there must be a centripetal force holding anything in circular motion (remember, circular motion is accelerated m ...
CHEMISTRY – UNITS 3 and 4 REVIEW PACKET Name Date
... 3. When an excited electron falls back to a lower energy, is it absorbing or emitting energy? _______________________________ ...
... 3. When an excited electron falls back to a lower energy, is it absorbing or emitting energy? _______________________________ ...
Periodic Trends
... occurs is related to the number of valence electrons. First ionization energy increases from left to right across a period. First ionization energy decreases down a group because atomic size increases and less energy is required to remove an electron farther from the nucleus. ...
... occurs is related to the number of valence electrons. First ionization energy increases from left to right across a period. First ionization energy decreases down a group because atomic size increases and less energy is required to remove an electron farther from the nucleus. ...
Laboratory Exercise: The Electronic Structure of the Hydrogen Atom
... Electromagnetic Spectrum of photon wavelengths is represented below: ...
... Electromagnetic Spectrum of photon wavelengths is represented below: ...
link to notes - UT-H GSBS Medical Physics Class Site
... – Electrons in orbits should repel each other making atom unstable, and – Electrons in circular orbits should radiate energy and spiral into nucleus ...
... – Electrons in orbits should repel each other making atom unstable, and – Electrons in circular orbits should radiate energy and spiral into nucleus ...
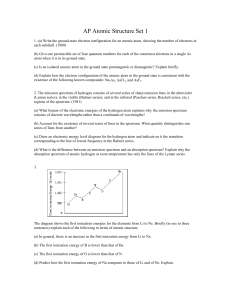
AP Atomic Structure Set 1
... 2. The emission spectrum of hydrogen consists of several series of sharp emission lines in the ultraviolet (Lyman series), in the visible (Balmer series), and in the infrared (Paschen series, Brackett series, etc,) regions of the spectrum. (1981) (a) What feature of the electronic energies of the hy ...
... 2. The emission spectrum of hydrogen consists of several series of sharp emission lines in the ultraviolet (Lyman series), in the visible (Balmer series), and in the infrared (Paschen series, Brackett series, etc,) regions of the spectrum. (1981) (a) What feature of the electronic energies of the hy ...
CHM111 Lab – Atomic Emission Spectroscopy – Grading Rubric
... When a high electrical potential is applied to a tube of hydrogen gas, the atoms will absorb some of the energy and reemit it as light. The distinct wavelengths emitted appear as lines when viewed through a spectroscope. Hydrogen emits light in the infrared, visible and ultrav ...
... When a high electrical potential is applied to a tube of hydrogen gas, the atoms will absorb some of the energy and reemit it as light. The distinct wavelengths emitted appear as lines when viewed through a spectroscope. Hydrogen emits light in the infrared, visible and ultrav ...
AP B - Unit 11 - 2013
... attraction to the nucleus (deflection is due to acceleration) - classical physics shows that any charged particle will radiate energy in the form of electromagnetic radiation when it is accelerated - quantum theory dictates that the radiation will be in the form of photons - since the radiated photo ...
... attraction to the nucleus (deflection is due to acceleration) - classical physics shows that any charged particle will radiate energy in the form of electromagnetic radiation when it is accelerated - quantum theory dictates that the radiation will be in the form of photons - since the radiated photo ...
Materialanalytik Praktikum UV-VIS Absorption B507
... A spectrophotometer can be either single beam or double beam. In a single beam instrument, Figure 3B, all of the light passes through the sample cell. However, in a doublebeam instrument, Figure 3C, the light is split into two beams before it reaches the sample. One beam is used as the reference; th ...
... A spectrophotometer can be either single beam or double beam. In a single beam instrument, Figure 3B, all of the light passes through the sample cell. However, in a doublebeam instrument, Figure 3C, the light is split into two beams before it reaches the sample. One beam is used as the reference; th ...
Kvantfysik Lecture Notes No. 4x
... The Bohr model strictly speaking only works for hydrogen or ions with a single electron. However, the Bohr model can still lead to very accurate predictions for certain experiments using multi-electron atoms. In particular, it leads to beautiful predictions for the x-ray spectra of atoms with Z > 15 ...
... The Bohr model strictly speaking only works for hydrogen or ions with a single electron. However, the Bohr model can still lead to very accurate predictions for certain experiments using multi-electron atoms. In particular, it leads to beautiful predictions for the x-ray spectra of atoms with Z > 15 ...
chapter10
... The best way to study the existence of the heaviest elements, nucleosynthesis in exploding stars, and other phenomena peculiar to the atomic nucleus is to create customized nuclei in an accelerator like Berkeley Lab's 88-Inch Cyclotron, then capture and analyze the gamma rays these nuclei emit when ...
... The best way to study the existence of the heaviest elements, nucleosynthesis in exploding stars, and other phenomena peculiar to the atomic nucleus is to create customized nuclei in an accelerator like Berkeley Lab's 88-Inch Cyclotron, then capture and analyze the gamma rays these nuclei emit when ...
o Lecturer: Dr. Peter Gallagher Email:
... 1. Fails describe why certain spectral lines are brighter than others => no mechanism for calculating transition probabilities. 2. Violates the uncertainty principal which dictates that position and momentum cannot be simultaneously determined. o Bohr model gives a basic conceptual model of elect ...
... 1. Fails describe why certain spectral lines are brighter than others => no mechanism for calculating transition probabilities. 2. Violates the uncertainty principal which dictates that position and momentum cannot be simultaneously determined. o Bohr model gives a basic conceptual model of elect ...
Lecture 15: Bohr Model of the Atom
... was not understood. • By the early 20th century, experiments showed that the protons were located within a very small volume called the nucleus, while the electrons moved about the nucleus in some complicated way. This was called the nuclear atom model. • Problem: Classical physics could not ex ...
... was not understood. • By the early 20th century, experiments showed that the protons were located within a very small volume called the nucleus, while the electrons moved about the nucleus in some complicated way. This was called the nuclear atom model. • Problem: Classical physics could not ex ...
I. Physical Biochemistry Protein Interactions Ultraviolet(UV
... Mathematically, each point of the protein spectra and each point of the ligand spectra are subtracted from the equivalent point of the protein-ligand spectra. The result is the UV absorbance associated with the conformational changes in the protein due to ligand target binding. ...
... Mathematically, each point of the protein spectra and each point of the ligand spectra are subtracted from the equivalent point of the protein-ligand spectra. The result is the UV absorbance associated with the conformational changes in the protein due to ligand target binding. ...
Name_______________________ Answers to Final Exam Study
... Produces hydrogen nuclei. b. The separation of one nucleus to form two nuclei of smaller mass c. Occurs when large nuclei fuse together. d. All of the above. 16. Which of the following types of radiation has negligible mass and does not change the number of protons or neutrons in an atom’s nucleus? ...
... Produces hydrogen nuclei. b. The separation of one nucleus to form two nuclei of smaller mass c. Occurs when large nuclei fuse together. d. All of the above. 16. Which of the following types of radiation has negligible mass and does not change the number of protons or neutrons in an atom’s nucleus? ...
投影片 1
... 2. It is the simplest bound state of nucleons and therefore gives us an ideal system for studying the nucleon-nucleon interaction. 3. An interesting feature of the deuteron is that it does not have excited states because it is a weakly bound system. ...
... 2. It is the simplest bound state of nucleons and therefore gives us an ideal system for studying the nucleon-nucleon interaction. 3. An interesting feature of the deuteron is that it does not have excited states because it is a weakly bound system. ...
Problem Set 1
... Atomic and Molecular Physics January -May 2010 Problem Sheet I 5th January 2010 due 15th January 2010 1. According the Bohr atom model what is the speed of an electron for the ground state of Hydrogen atom? 2.Consider the absorption or emission of photon of energy hν by an atom initially at rest.Aft ...
... Atomic and Molecular Physics January -May 2010 Problem Sheet I 5th January 2010 due 15th January 2010 1. According the Bohr atom model what is the speed of an electron for the ground state of Hydrogen atom? 2.Consider the absorption or emission of photon of energy hν by an atom initially at rest.Aft ...
Chpater 5.3 PPT
... Larger atomic radius than atom Due to electrons being added decreased effective nuclear charge Greater repulsion of electrons ...
... Larger atomic radius than atom Due to electrons being added decreased effective nuclear charge Greater repulsion of electrons ...
Problem sets 09-20-2..
... 2a) The IR absorption spectrum of HCl gives rise to a P, Q, and R branch. What are the selection rules that govern each of these branches in the mid IR and far IR spectral region? 2b) List the quantum numbers that characterize the ground and the excited state of the P(2), and R(3) transition in the ...
... 2a) The IR absorption spectrum of HCl gives rise to a P, Q, and R branch. What are the selection rules that govern each of these branches in the mid IR and far IR spectral region? 2b) List the quantum numbers that characterize the ground and the excited state of the P(2), and R(3) transition in the ...
Mössbauer spectroscopy

Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer in 1957, consists in the recoil-free, resonant absorption and emission of gamma rays in solids.Like NMR spectroscopy, Mössbauer spectroscopy probes tiny changes in the energy levels of an atomic nucleus in response to its environment. Typically, three types of nuclear interactions may be observed: an isomeric shift, also known as a chemical shift; quadrupole splitting; and magnetic or hyperfine splitting, also known as the Zeeman effect. Due to the high energy and extremely narrow line widths of gamma rays, Mössbauer spectroscopy is a very sensitive technique in terms of energy (and hence frequency) resolution, capable of detecting change in just a few parts per 1011.