UV-Vis (electronic) spectroscopy
... – Relaxed by effects that make spin a poor quantum number (heavy atoms) • Symmetry-forbidden transitions – Transitions between states of the same parity are forbidden – Particularly important for centro-symmetric molecules (ethene) – Relaxed by coupling of electronic transitions to vibrational trans ...
... – Relaxed by effects that make spin a poor quantum number (heavy atoms) • Symmetry-forbidden transitions – Transitions between states of the same parity are forbidden – Particularly important for centro-symmetric molecules (ethene) – Relaxed by coupling of electronic transitions to vibrational trans ...
CERN workshop 2015
... Göran Andersson (one of the founders of ISOLDE) retired and was succeeded by Björn Jonson Björn gave me the isotope separator LILLJON I could start investigating negative ions ...
... Göran Andersson (one of the founders of ISOLDE) retired and was succeeded by Björn Jonson Björn gave me the isotope separator LILLJON I could start investigating negative ions ...
Chapter 1 Chemistry: The Study of Matter
... There are more Plasma – high temperature low pressure – electrons separate from nucleus – Most common in the universe More at very low temp – Bose- Einstein condensate – Quantum superfluids ...
... There are more Plasma – high temperature low pressure – electrons separate from nucleus – Most common in the universe More at very low temp – Bose- Einstein condensate – Quantum superfluids ...
bp 811 et. advanced instrumentation techniques
... BP 811 ET. ADVANCED INSTRUMENTATION TECHNIQUES 45 Hours Scope: This subject deals with the application of instrumental methods in qualitative and quantitative analysis of drugs. This subject is designed to impart advanced knowledge on the principles and instrumentation of spectroscopic and chromatog ...
... BP 811 ET. ADVANCED INSTRUMENTATION TECHNIQUES 45 Hours Scope: This subject deals with the application of instrumental methods in qualitative and quantitative analysis of drugs. This subject is designed to impart advanced knowledge on the principles and instrumentation of spectroscopic and chromatog ...
Electronic Spectroscopy of Transition Metal Ions
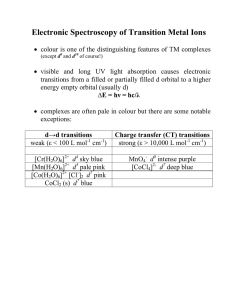
... in order to understand the spectroscopy of d ions with more than one d electron we must take the effect of e- - e- repulsion into account (we have ignored this so far) ...
... in order to understand the spectroscopy of d ions with more than one d electron we must take the effect of e- - e- repulsion into account (we have ignored this so far) ...
NMR SPECTROSCOPY
... 2) The sample is irradiated with a range of radio frequency light to transfer nuclei from the lower to the higher energy state. 3) The oscillating magnetic fields produced by the nuclei are observed using the same coil that was used for the irradiation. A complex, decaying signal is observed that co ...
... 2) The sample is irradiated with a range of radio frequency light to transfer nuclei from the lower to the higher energy state. 3) The oscillating magnetic fields produced by the nuclei are observed using the same coil that was used for the irradiation. A complex, decaying signal is observed that co ...
Setting the stage
... five atoms, 15 with six atoms, 9 with seven atoms, 10 with eight atoms, 9 with nine atoms, 15 with ten or more atoms and 17 deuterated molecules. 204 different molecules altogether who‘s ingredients include H, C, N, O, F, Na, Mg, Al, Si, P, S, Cl, K, Ti, Fe, I. ...
... five atoms, 15 with six atoms, 9 with seven atoms, 10 with eight atoms, 9 with nine atoms, 15 with ten or more atoms and 17 deuterated molecules. 204 different molecules altogether who‘s ingredients include H, C, N, O, F, Na, Mg, Al, Si, P, S, Cl, K, Ti, Fe, I. ...
Document
... Better resolution at higher frequencies(say Q - band) But Higher Intensitiesat lower fields. (say s - band) ...
... Better resolution at higher frequencies(say Q - band) But Higher Intensitiesat lower fields. (say s - band) ...
Spectrometry 1 R
... of light transmitted and thus causing positive deviations. • Deviations in absorptive coefficients at high concentrations (>0.01M) due to electrostatic interactions between molecules in close proximity. • Stray light ; it is the quantity of light that reaches the detector that is of a wavelength oth ...
... of light transmitted and thus causing positive deviations. • Deviations in absorptive coefficients at high concentrations (>0.01M) due to electrostatic interactions between molecules in close proximity. • Stray light ; it is the quantity of light that reaches the detector that is of a wavelength oth ...
Introduction to Quantum Mechanics and Multiplet Splitting in 1H
... difficult even for some faculty. As a result, many students in undergraduate chemistry courses are never exposed to these two important topics. In this classroom demonstration we will simplify the explanation and make the abstract concepts more concrete by combining quantum mechanics and NMR spectro ...
... difficult even for some faculty. As a result, many students in undergraduate chemistry courses are never exposed to these two important topics. In this classroom demonstration we will simplify the explanation and make the abstract concepts more concrete by combining quantum mechanics and NMR spectro ...
SpectraPart2
... Given a model of the atom showing several energy levels, identify which photon comes from which electron transition. (See the tutorial.) What information can astronomers obtain from the spectrum of a star, galaxy or gas cloud? What did Annie Cannon contribute to the study of spectra? Cecilia Payne? ...
... Given a model of the atom showing several energy levels, identify which photon comes from which electron transition. (See the tutorial.) What information can astronomers obtain from the spectrum of a star, galaxy or gas cloud? What did Annie Cannon contribute to the study of spectra? Cecilia Payne? ...
Nuclear Chemistry Review
... particles, beta particles, or gamma radiation from the nucleus of an unstable isotope. These emissions differ in mass, charge, and penetrating power. • Be able to recognize and write the symbols for all of the particles and radiation emitted from radioactive nuclides – they’re all found on Table O. ...
... particles, beta particles, or gamma radiation from the nucleus of an unstable isotope. These emissions differ in mass, charge, and penetrating power. • Be able to recognize and write the symbols for all of the particles and radiation emitted from radioactive nuclides – they’re all found on Table O. ...
Unit 5 – Test Study Guide
... For example: Ionization energy decreases down a column because with the addition of another energy level and many more inner core electrons the atoms is much bigger. This means the valence electrons are further away from the nucleus and they are less attracted to the nucleus due to all the inner cor ...
... For example: Ionization energy decreases down a column because with the addition of another energy level and many more inner core electrons the atoms is much bigger. This means the valence electrons are further away from the nucleus and they are less attracted to the nucleus due to all the inner cor ...
cp351c04
... decays to lower states, with emission of photon (or other mechanism for energy transfer). Metastable State: “sort of stable” state state with a longer life time than ordinary excited states lifetime ~ 1E-3 s vs. 1E-8 s for ordinary states Three kinds of transitions h ...
... decays to lower states, with emission of photon (or other mechanism for energy transfer). Metastable State: “sort of stable” state state with a longer life time than ordinary excited states lifetime ~ 1E-3 s vs. 1E-8 s for ordinary states Three kinds of transitions h ...
Chapter 2 - Las Positas College
... Case (b) does nor represent a possible electron configuration since it has three electrons in the 2s state and only two are allowed. Case (c) represents a possible electron configuration for an atom with five electrons (Boron) in an excited state and the configuration is 1s2 2p3. ...
... Case (b) does nor represent a possible electron configuration since it has three electrons in the 2s state and only two are allowed. Case (c) represents a possible electron configuration for an atom with five electrons (Boron) in an excited state and the configuration is 1s2 2p3. ...
Quantum optics with gamma radiation
... Even if a solid material could be prepared with most of the nuclei in the ground state as well as a large number in some longlived isomeric state, without having an inverted system, lasing might still be realised. To obtain lasing from such a system, the concept of "lasing without inversion" as intr ...
... Even if a solid material could be prepared with most of the nuclei in the ground state as well as a large number in some longlived isomeric state, without having an inverted system, lasing might still be realised. To obtain lasing from such a system, the concept of "lasing without inversion" as intr ...
l - Evergreen
... Look at Fig.7.4. Predict the probability (without calculating) that the electron in the (n,l) = (2,0) state is found inside the Bohr radius. ...
... Look at Fig.7.4. Predict the probability (without calculating) that the electron in the (n,l) = (2,0) state is found inside the Bohr radius. ...
Honors Midterm - Stamford High School
... 40/5 = 8 days is one half life for I-131 19. Draw and label how alpha( , beta (, and gamma ( rays each travel when they pass through an electric field. Use the diagram below to illustrate your answer. What direction does each particle travel and what bending occurs. ...
... 40/5 = 8 days is one half life for I-131 19. Draw and label how alpha( , beta (, and gamma ( rays each travel when they pass through an electric field. Use the diagram below to illustrate your answer. What direction does each particle travel and what bending occurs. ...
Introduction to Atomic Spectroscopy
... irradiated with a monochromatic beam of radiation of enough energy to cause electronic excitation, emission takes place in all directions. The emitted radiation from the first excited electronic level, collected at 90o to the incident beam, is called resonance fluorescence. Photons of the same wavel ...
... irradiated with a monochromatic beam of radiation of enough energy to cause electronic excitation, emission takes place in all directions. The emitted radiation from the first excited electronic level, collected at 90o to the incident beam, is called resonance fluorescence. Photons of the same wavel ...
7.3-Flame Test Lab
... All metal elements have distinct properties. One of these properties is its own special color when burned in an open flame. This is caused by the flame exciting the electrons of the metal element in a compound. When the electrons return from the excited state to the ground state, a photon of energy ...
... All metal elements have distinct properties. One of these properties is its own special color when burned in an open flame. This is caused by the flame exciting the electrons of the metal element in a compound. When the electrons return from the excited state to the ground state, a photon of energy ...
Communicating Research to the General Public
... don’t know. We aren’t sure which Mn atoms accept the charge, or why. We don’t know much about the evolution of the structure from one step to the next, or which parts of the chemical design are most critical. Until we know these things, it will be difficult to mimic this design artificially. The OEC ...
... don’t know. We aren’t sure which Mn atoms accept the charge, or why. We don’t know much about the evolution of the structure from one step to the next, or which parts of the chemical design are most critical. Until we know these things, it will be difficult to mimic this design artificially. The OEC ...
Advantages of FTIR spectroscopy
... mid-infrared spectroscopy is is of the Fourier transform type. This is the reason why only FTIR technology will be described in the following. Bruker Optics has specialized in the field of FT-IR spectroscopy since 1974, and is one of the leading manufacturers of FT-IR, FT-NIR and FT-Raman spectromet ...
... mid-infrared spectroscopy is is of the Fourier transform type. This is the reason why only FTIR technology will be described in the following. Bruker Optics has specialized in the field of FT-IR spectroscopy since 1974, and is one of the leading manufacturers of FT-IR, FT-NIR and FT-Raman spectromet ...
Chemistry 4021/8021 Computational Chemistry 3/4 Credits Spring
... level by 95.9%. (Note, incidentally, that there is no 6-31G(d) basis set for Ni, but G03 by convention uses a so-called McClean-Chandler basis for the firstrow transition metals when 6-31G(d) is listed in the keyword line. Note also that there is no guarantee that we should expect our nickel systems ...
... level by 95.9%. (Note, incidentally, that there is no 6-31G(d) basis set for Ni, but G03 by convention uses a so-called McClean-Chandler basis for the firstrow transition metals when 6-31G(d) is listed in the keyword line. Note also that there is no guarantee that we should expect our nickel systems ...
nuclear chemistry - Wood County Schools
... Beta Decay: Medium-level radiation from the emission of beta particles (electrons). Positron Emission: Medium-level radiation from the emission of a positron, which is the same as an electron, only with a positive charge, converting a proton into a neutron. Electron Capture: When an atom takes in an ...
... Beta Decay: Medium-level radiation from the emission of beta particles (electrons). Positron Emission: Medium-level radiation from the emission of a positron, which is the same as an electron, only with a positive charge, converting a proton into a neutron. Electron Capture: When an atom takes in an ...
Mössbauer spectroscopy

Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer in 1957, consists in the recoil-free, resonant absorption and emission of gamma rays in solids.Like NMR spectroscopy, Mössbauer spectroscopy probes tiny changes in the energy levels of an atomic nucleus in response to its environment. Typically, three types of nuclear interactions may be observed: an isomeric shift, also known as a chemical shift; quadrupole splitting; and magnetic or hyperfine splitting, also known as the Zeeman effect. Due to the high energy and extremely narrow line widths of gamma rays, Mössbauer spectroscopy is a very sensitive technique in terms of energy (and hence frequency) resolution, capable of detecting change in just a few parts per 1011.