isotopes and atomic mass
... 1. Which of the data in the table must be measured and which must be calculated? 2. In all except step 11, the “Total” is calculated by adding the numbers across each row. Step 11 is an exception because it does not take into account the fact that there are different numbers of each isotope. Rather ...
... 1. Which of the data in the table must be measured and which must be calculated? 2. In all except step 11, the “Total” is calculated by adding the numbers across each row. Step 11 is an exception because it does not take into account the fact that there are different numbers of each isotope. Rather ...
Study Island
... properties and belong to the same family of elements. Valence electrons are the outermost electrons in the atom and are important in determining how the atom chemically reacts with other atoms. 2. Matter is conserved during a chemical reaction, which means that the number of atoms involved in the re ...
... properties and belong to the same family of elements. Valence electrons are the outermost electrons in the atom and are important in determining how the atom chemically reacts with other atoms. 2. Matter is conserved during a chemical reaction, which means that the number of atoms involved in the re ...
Atomic terms Example: Helium has an atomic number of 2. Every
... Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name of the element. Sometimes, the abbreviation is based on a language OTHER THAN ENGLISH! (Example: Na is short for "natrium", the Latin name of s ...
... Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name of the element. Sometimes, the abbreviation is based on a language OTHER THAN ENGLISH! (Example: Na is short for "natrium", the Latin name of s ...
Chemistry Review- Answer all questions on loose
... c) Sodium or Magnesium - Both Na and Mg are in the same period on the periodic table, but Na has only one valence electron (group 1-Alkali metals) making it violently reactive. Na is very close to a full electron shell, it only has to lose one electron and that will happen more readily than Mg losin ...
... c) Sodium or Magnesium - Both Na and Mg are in the same period on the periodic table, but Na has only one valence electron (group 1-Alkali metals) making it violently reactive. Na is very close to a full electron shell, it only has to lose one electron and that will happen more readily than Mg losin ...
Unit 2 Lesson 1 - Mrs. Tainter`s Physical Science Class
... proton, found in nuclei of atoms along with protons the center of positive charge called protons – also contains protons (with no charge) the sum of the number of protons and the number of neutrons in a single atom. the number of protons in every atom of an element. atoms of the same element with di ...
... proton, found in nuclei of atoms along with protons the center of positive charge called protons – also contains protons (with no charge) the sum of the number of protons and the number of neutrons in a single atom. the number of protons in every atom of an element. atoms of the same element with di ...
Ch. 4 Slides
... system) does each of the following elements belong? If the group has a name, indicate that as well. ...
... system) does each of the following elements belong? If the group has a name, indicate that as well. ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... Groups Each group is also called a “family” of elements. Just like members of the same family, they share similar characteristics. Each element family has a unique name as well! Let’s look at them now… ...
... Groups Each group is also called a “family” of elements. Just like members of the same family, they share similar characteristics. Each element family has a unique name as well! Let’s look at them now… ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... Groups Each group is also called a “family” of elements. Just like members of the same family, they share similar characteristics. Each element family has a unique name as well! Let’s look at them now… ...
... Groups Each group is also called a “family” of elements. Just like members of the same family, they share similar characteristics. Each element family has a unique name as well! Let’s look at them now… ...
Unit 2 Atomic Theory
... Half-Life (t1/2) - time required for one half of the original sample of nuclei to decay. •The half-life of Ra-223 is 12 days. If you start with 100.0 grams of Ra-223, how much will be left after 36 days? 100.0 g 50.00 g 25.00 g 12.50 g •The half life of Ra-225 is 15 minutes. If you have 10.0 ...
... Half-Life (t1/2) - time required for one half of the original sample of nuclei to decay. •The half-life of Ra-223 is 12 days. If you start with 100.0 grams of Ra-223, how much will be left after 36 days? 100.0 g 50.00 g 25.00 g 12.50 g •The half life of Ra-225 is 15 minutes. If you have 10.0 ...
Atomic Structure - Learn District 196
... • If you change the number of protons, you change the type of atom itself. • If you change the number of electrons, you change the atom to an ion (charged particle). • If you change the number of neutrons, you change the isotope of that element. ...
... • If you change the number of protons, you change the type of atom itself. • If you change the number of electrons, you change the atom to an ion (charged particle). • If you change the number of neutrons, you change the isotope of that element. ...
Atomic Structure
... • If you change the number of protons, you change the type of atom itself. • If you change the number of electrons, you change the atom to an ion (charged particle). • If you change the number of neutrons, you change the isotope of that element. ...
... • If you change the number of protons, you change the type of atom itself. • If you change the number of electrons, you change the atom to an ion (charged particle). • If you change the number of neutrons, you change the isotope of that element. ...
Atoms, Molecules and Ions Part 2
... element must have the same mass. • One of the 2 flaws in Dalton’s theory was, atoms of the same element can have DIFFERENT masses. • The mass of an atom is due to the mass of the protons and the neutrons in that atom. • Isotopes are atoms of the same element which have the same number of protons but ...
... element must have the same mass. • One of the 2 flaws in Dalton’s theory was, atoms of the same element can have DIFFERENT masses. • The mass of an atom is due to the mass of the protons and the neutrons in that atom. • Isotopes are atoms of the same element which have the same number of protons but ...
Atomic Structure Worksheet
... Look at the atomic weights of a few different elements on your periodic table. Do you notice that very few of the elements have atomic weights that are close to being nice whole numbers? Do you know why this is? After all, for our purposes, the mass of both the proton and the neutron are almost exac ...
... Look at the atomic weights of a few different elements on your periodic table. Do you notice that very few of the elements have atomic weights that are close to being nice whole numbers? Do you know why this is? After all, for our purposes, the mass of both the proton and the neutron are almost exac ...
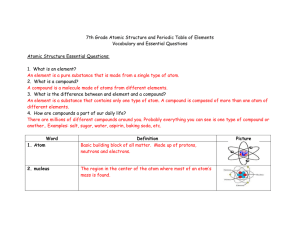
7th Grade Atomic Structure and Periodic Table of Elements
... A compound is a molecule made of atoms from different elements. 3. What is the difference between and element and a compound? An element is a substance that contains only one type of atom. A compound is composed of more than one atom of different elements. 4. How are compounds a part of our daily li ...
... A compound is a molecule made of atoms from different elements. 3. What is the difference between and element and a compound? An element is a substance that contains only one type of atom. A compound is composed of more than one atom of different elements. 4. How are compounds a part of our daily li ...
Atomic Structure and Periodic Table Review Guide
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
ATOMIC THEORY WORKSHEET 1. Which of the following
... reactions the old bonds between atoms are broken down and new bonds are formed. Atoms, however, can be created or destroyed in nuclear reactions: radioactive decays, nuclear fission and fusion. ...
... reactions the old bonds between atoms are broken down and new bonds are formed. Atoms, however, can be created or destroyed in nuclear reactions: radioactive decays, nuclear fission and fusion. ...
ATOMIC THEORY WORKSHEET 1.
... reactions the old bonds between atoms are broken down and new bonds are formed. Atoms, however, can be created or destroyed in nuclear reactions: radioactive decays, nuclear fission and fusion. ...
... reactions the old bonds between atoms are broken down and new bonds are formed. Atoms, however, can be created or destroyed in nuclear reactions: radioactive decays, nuclear fission and fusion. ...
Document
... Dalton’s Atomic Theory - Summary 1. matter is composed, indivisible particles (atoms) 2. all atoms of a particular element are identical 3. different elements have different atoms 4. atoms combine in certain whole-number ratios 5. In a chemical reaction, atoms are merely rearranged to form new comp ...
... Dalton’s Atomic Theory - Summary 1. matter is composed, indivisible particles (atoms) 2. all atoms of a particular element are identical 3. different elements have different atoms 4. atoms combine in certain whole-number ratios 5. In a chemical reaction, atoms are merely rearranged to form new comp ...
document
... • Copper has two isotopes: 63Cu with a mass of 63.01 amu and an abundance of 69% and 65Cu with a mass of 65.01 amu and an abundance of 31%. Calculate the atomic mass of copper. • In 100 g of copper, how many grams of 63Cu? ...
... • Copper has two isotopes: 63Cu with a mass of 63.01 amu and an abundance of 69% and 65Cu with a mass of 65.01 amu and an abundance of 31%. Calculate the atomic mass of copper. • In 100 g of copper, how many grams of 63Cu? ...
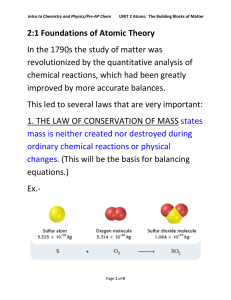
2:1 Foundations of Atomic Theory In the 1790s the study of matter
... of atoms in an element in a sample with a known mass. Atoms of different elements have different numbers of protons; Atoms of the same element have the same number of protons. The ATOMIC NUMBER (Z) of an element is the number of protons of each atom of that element, so it identifies an element. The ...
... of atoms in an element in a sample with a known mass. Atoms of different elements have different numbers of protons; Atoms of the same element have the same number of protons. The ATOMIC NUMBER (Z) of an element is the number of protons of each atom of that element, so it identifies an element. The ...
Atomic Structure Notes
... them) is called a period Each vertical column is called a group, or family Elements in a group have similar chemical and physical properties Identified with a number and either an “A” or “B” More presented in Chapter 6 ...
... them) is called a period Each vertical column is called a group, or family Elements in a group have similar chemical and physical properties Identified with a number and either an “A” or “B” More presented in Chapter 6 ...
Modern Physics
... • Above element 83, the protons are farther and farther away from each other in the nucleus, therefore requiring a greater nuclear force to overcome the electrostatic repulsive forces. The nuclear force is not strong enough to hold together the protons when more than 83 of them are packed into a nuc ...
... • Above element 83, the protons are farther and farther away from each other in the nucleus, therefore requiring a greater nuclear force to overcome the electrostatic repulsive forces. The nuclear force is not strong enough to hold together the protons when more than 83 of them are packed into a nuc ...