Monomer - Teacher Pages
... What’s the big deal about CARBON? • Carbon easily forms strong nonpolar covalent bonds with other atoms, including other carbon atoms. • Each carbon creates four bonds to other atoms (some of which may be other carbon atoms), giving carbon structures diversity, flexibility, and strength. • Carbons ...
... What’s the big deal about CARBON? • Carbon easily forms strong nonpolar covalent bonds with other atoms, including other carbon atoms. • Each carbon creates four bonds to other atoms (some of which may be other carbon atoms), giving carbon structures diversity, flexibility, and strength. • Carbons ...
(Lecture(21) - MSU Chemistry
... 1. be'familiar'with'the'properties'of'and'bonding'in'the'different'allotropes'of'carbon.' 2. know'the'different'types'of'compounds'formed'by'carbon'and'be'able'to'classify' carbon'compounds.' 3. be'able'to'determine'the'oxidation'number'of'C'in'carbon'compounds.' 4. know'the'relationship'between'hyb ...
... 1. be'familiar'with'the'properties'of'and'bonding'in'the'different'allotropes'of'carbon.' 2. know'the'different'types'of'compounds'formed'by'carbon'and'be'able'to'classify' carbon'compounds.' 3. be'able'to'determine'the'oxidation'number'of'C'in'carbon'compounds.' 4. know'the'relationship'between'hyb ...
Practice Bypass Answers
... to obtain bent shape; also, oxygen has significantly higher electronegativity (attraction for shared electrons) than hydrogen 3.5 vs 2.2, which causes permanent shift of electron cloud towards oxygen and as a result formation of partial weak negative charge on the oxygen end, δ – , and partial weak ...
... to obtain bent shape; also, oxygen has significantly higher electronegativity (attraction for shared electrons) than hydrogen 3.5 vs 2.2, which causes permanent shift of electron cloud towards oxygen and as a result formation of partial weak negative charge on the oxygen end, δ – , and partial weak ...
Chapter 4 REVIEW
... 28. Chlorine is a very reactive element that forms stable compounds with most other elements. For each of the following chlorine compounds, draw Lewis and structural diagrams, and then predict the polarity of the molecules: (a) NCl3 (c) PCl5 (b) SiCl4 (d) SCl6 ...
... 28. Chlorine is a very reactive element that forms stable compounds with most other elements. For each of the following chlorine compounds, draw Lewis and structural diagrams, and then predict the polarity of the molecules: (a) NCl3 (c) PCl5 (b) SiCl4 (d) SCl6 ...
EXAM 1-A fall 2004.doc
... j. the science, or study of life k. a molecule of two or more atoms of different kinds l. a group of similar organisms that are capable of interbreeding under natural conditions m. an educated guess attempting to explain observations n. independently worked out the Theory of Evolution while in the F ...
... j. the science, or study of life k. a molecule of two or more atoms of different kinds l. a group of similar organisms that are capable of interbreeding under natural conditions m. an educated guess attempting to explain observations n. independently worked out the Theory of Evolution while in the F ...
SECTION B Part 2
... period 2 (C, O, N) using 2p orbitals. • e.g. C=C, C=O, O2, N2, N=O • 2s/2p orbitals are similar in size and energy and therefore “hybridize” well • Mixing of 2s/2p orbitals on adjacent atoms is highly efficient (small and localized due to high Zeff) and form strong bonds • Not for period 3 and below ...
... period 2 (C, O, N) using 2p orbitals. • e.g. C=C, C=O, O2, N2, N=O • 2s/2p orbitals are similar in size and energy and therefore “hybridize” well • Mixing of 2s/2p orbitals on adjacent atoms is highly efficient (small and localized due to high Zeff) and form strong bonds • Not for period 3 and below ...
Stereocenter www.AssignmentPoint.com A stereocenter or
... there are four different groups attached to the carbon atom. ...
... there are four different groups attached to the carbon atom. ...
Name
... . There is also a fourth and fifth phase,They are and , but they exist at very high temperatures. Science Is Fun Go to the “ChemTime Clock” area to find the answers to these questions. 1. All materials, whether solid, liquid or gas, are made of . Atoms are the smallest of . Scientists have found ove ...
... . There is also a fourth and fifth phase,They are and , but they exist at very high temperatures. Science Is Fun Go to the “ChemTime Clock” area to find the answers to these questions. 1. All materials, whether solid, liquid or gas, are made of . Atoms are the smallest of . Scientists have found ove ...
HiQ VERISEQ Carbon dioxide
... To be approved by the United States (US) Food and Drug Administration (FDA) as a manufacturer of active pharmaceutical ingredients (APIs) or pharmaceutical drug products, full compliance with current Good Manufacturing Practice (cGMP) should be assured. With gases used in pharmaceutical production, ...
... To be approved by the United States (US) Food and Drug Administration (FDA) as a manufacturer of active pharmaceutical ingredients (APIs) or pharmaceutical drug products, full compliance with current Good Manufacturing Practice (cGMP) should be assured. With gases used in pharmaceutical production, ...
VERISEQ® pharmaceutical grade gases. Carbon→dioxide.
... pharmaceutical ingredients (APIs) or pharmaceutical drug products, full compliance with current Good Manufacturing Practice (cGMP) should be assured. With gases used in pharmaceutical production, producers need to fulfil the requirements of US FDA Title 21 Code of Federal Regulations (CFR) Parts 210 ...
... pharmaceutical ingredients (APIs) or pharmaceutical drug products, full compliance with current Good Manufacturing Practice (cGMP) should be assured. With gases used in pharmaceutical production, producers need to fulfil the requirements of US FDA Title 21 Code of Federal Regulations (CFR) Parts 210 ...
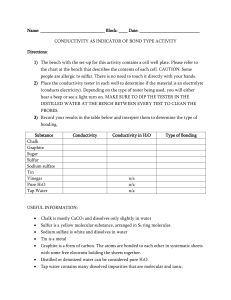
Conductivity as an Indicator of Bond Type
... Sulfur is a yellow molecular substance, arranged in S8 ring molecules ...
... Sulfur is a yellow molecular substance, arranged in S8 ring molecules ...
the teeni tiny atoms - Supercomputing Challenge
... Diamonds are made of carbon. Each carbon atom is attached to four other carbon atoms with tight bonds. There are three types of carbon Diamond, Graphite, and The Buckminster fullerene. Diamond is the hardest natural material known to man, Graphite has a special crystalline structure with the carbon ...
... Diamonds are made of carbon. Each carbon atom is attached to four other carbon atoms with tight bonds. There are three types of carbon Diamond, Graphite, and The Buckminster fullerene. Diamond is the hardest natural material known to man, Graphite has a special crystalline structure with the carbon ...
Atomic number.
... • All living beings are organized. The smallest part of a living organism is the atom. Atoms are the smallest part of an element. An element is a pure substance. • There are 25 different elements necessary to life can be classiffied into: SPONCH (98%) and Trace elements (elements that the body need ...
... • All living beings are organized. The smallest part of a living organism is the atom. Atoms are the smallest part of an element. An element is a pure substance. • There are 25 different elements necessary to life can be classiffied into: SPONCH (98%) and Trace elements (elements that the body need ...
TM - Intro to Organi..
... • Without this property, large biomolecules such as proteins, lipids, carbohydrates, and nucleic acids could not form. • Carbon easily forms bonds with other non-metal atoms. ...
... • Without this property, large biomolecules such as proteins, lipids, carbohydrates, and nucleic acids could not form. • Carbon easily forms bonds with other non-metal atoms. ...
Practice Problems
... • Making potassium nitride from its component elements • Uranium (VI) fluoride reacts with magnesium metal ...
... • Making potassium nitride from its component elements • Uranium (VI) fluoride reacts with magnesium metal ...
Name_________________________________
... Go to http://sciencespot.net/ and click the Kid Zone graphic! Part 2: Go to the “Matter and Atoms” Section under Chemistry. Click on “Science is Fun” under General Sites. Go to the “ChemTime Clock” area to find the answers. 1) All materials, whether solid, liquid or gas, are made of ____________. ...
... Go to http://sciencespot.net/ and click the Kid Zone graphic! Part 2: Go to the “Matter and Atoms” Section under Chemistry. Click on “Science is Fun” under General Sites. Go to the “ChemTime Clock” area to find the answers. 1) All materials, whether solid, liquid or gas, are made of ____________. ...
53 word equations
... The new substances which are produced are called the products. In a word equation: ...
... The new substances which are produced are called the products. In a word equation: ...
The ATOM - Aarmstrongchem
... exactly the same proportions regardless of the size of the sample or the source ...
... exactly the same proportions regardless of the size of the sample or the source ...
Chapter 3 PowerPoint
... Aristotle, however, did not believe in atoms. He thought all matter was continuous. Democritus does not get credit for discovering the atom because he had no scientific evidence to back it up. ...
... Aristotle, however, did not believe in atoms. He thought all matter was continuous. Democritus does not get credit for discovering the atom because he had no scientific evidence to back it up. ...
Atomic Structure Video Guide
... 20. The periodic table arranges elements by their atomic ___________________. 21. Calcium is an element found in ______________, __________________ and bones. 22. There are 18 columns known as _________________ or ________________. The rows are called ___________. 23. As you move from left to right ...
... 20. The periodic table arranges elements by their atomic ___________________. 21. Calcium is an element found in ______________, __________________ and bones. 22. There are 18 columns known as _________________ or ________________. The rows are called ___________. 23. As you move from left to right ...
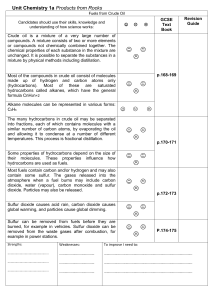
Unit_Chemistry_1a_Oil
... Crude oil is a mixture of a very large number of compounds. A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged. It is possible to separate the substances in a mixture by physical methods inc ...
... Crude oil is a mixture of a very large number of compounds. A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged. It is possible to separate the substances in a mixture by physical methods inc ...
Structure of the Atom
... Information: Structure of the Atom Note the following symbols: (they are not to scale) = proton (positive charge) = electron (negative charge) = neutron (no charge) The following three diagrams are hydrogen atoms: ...
... Information: Structure of the Atom Note the following symbols: (they are not to scale) = proton (positive charge) = electron (negative charge) = neutron (no charge) The following three diagrams are hydrogen atoms: ...
Allotropes of carbon

Carbon is capable of forming many allotropes due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades many more allotropes and forms of carbon have been discovered and researched including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperature or extreme pressures.