module 1: metal carbonyls
... prepared numerous compounds containing metal and carbon monoxide. Compounds having at least one bond between carbon and metal are known as organometallic compounds. Metal carbonyls are the transition metal complexes of carbon monoxide containing metal-carbon bond. Lone pair of electrons are availabl ...
... prepared numerous compounds containing metal and carbon monoxide. Compounds having at least one bond between carbon and metal are known as organometallic compounds. Metal carbonyls are the transition metal complexes of carbon monoxide containing metal-carbon bond. Lone pair of electrons are availabl ...
P-BLOCK ELEMENTS
... molecule has 60 vertices. It contains both single (143.5) and double (138.3) bonds. It has cage like structure like a soccer ball having hexagonal and pentagonal rings of carbon. It has 20-six membered ring and 12 five membered rings. Carbon atom is sp2 hydridised. Remaining electron is delocalised ...
... molecule has 60 vertices. It contains both single (143.5) and double (138.3) bonds. It has cage like structure like a soccer ball having hexagonal and pentagonal rings of carbon. It has 20-six membered ring and 12 five membered rings. Carbon atom is sp2 hydridised. Remaining electron is delocalised ...
Carbon Chemistry - North Allegheny School District
... compound that contains only carbon and hydrogen atoms is called a hydrocarbon. The simplest hydrocarbon is methane, the primary component of natural gas. If you have a gas stove or gas furnace in your home, methane usually is the fuel that is burned in these appliances. Methane consists of a single ...
... compound that contains only carbon and hydrogen atoms is called a hydrocarbon. The simplest hydrocarbon is methane, the primary component of natural gas. If you have a gas stove or gas furnace in your home, methane usually is the fuel that is burned in these appliances. Methane consists of a single ...
Metallic bonding
... Covalent Crystals : Diamond, graphite and Quartz In giant covalent crystals. atoms are bonded by strong covalent bonds only. The properties of the crystals are governed by the geometry and the strength of the bonds. Diamond and graphite are allotropes of carbon. They have different structures. ...
... Covalent Crystals : Diamond, graphite and Quartz In giant covalent crystals. atoms are bonded by strong covalent bonds only. The properties of the crystals are governed by the geometry and the strength of the bonds. Diamond and graphite are allotropes of carbon. They have different structures. ...
STRUCTURES OF CRYSTALS
... Covalent Crystals : Diamond, graphite and Quartz In giant covalent crystals. atoms are bonded by strong covalent bonds only. The properties of the crystals are governed by the geometry and the strength of the bonds. Diamond and graphite are allotropes of carbon. They have different structures. ...
... Covalent Crystals : Diamond, graphite and Quartz In giant covalent crystals. atoms are bonded by strong covalent bonds only. The properties of the crystals are governed by the geometry and the strength of the bonds. Diamond and graphite are allotropes of carbon. They have different structures. ...
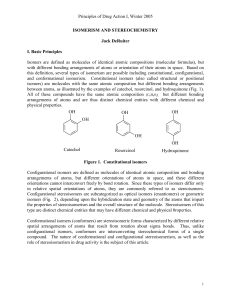
Principles of Drug Action I, Winter 2005 ISOMERISM AND
... that chemically identical salts of tartaric acid rotated plane-polarized light differently. Pasteur discovered that two distinct crystalline forms of tartaric acid salt could be obtained from solutions of the optically inactive salt of "paratartaric acid" (also known as racemic acid), and that one c ...
... that chemically identical salts of tartaric acid rotated plane-polarized light differently. Pasteur discovered that two distinct crystalline forms of tartaric acid salt could be obtained from solutions of the optically inactive salt of "paratartaric acid" (also known as racemic acid), and that one c ...
索书号:O62 /C713p (MIT) Principles and Applications Of
... 13 Synthetic Applications of Transition-Metal Hydrides 14 Synthetic Applications of Complexes Containing Metal-Carbon σBonds 15 Synthetic Applications of Transition-Metal Carbonyl Compounds 16 Synthetic Applications of Transition-Metal Carbenes and Metallacycles 17 Synthetic Applications of Transiti ...
... 13 Synthetic Applications of Transition-Metal Hydrides 14 Synthetic Applications of Complexes Containing Metal-Carbon σBonds 15 Synthetic Applications of Transition-Metal Carbonyl Compounds 16 Synthetic Applications of Transition-Metal Carbenes and Metallacycles 17 Synthetic Applications of Transiti ...
Hybridization and St..
... The steric number is determined by counting the number of bonded groups attached to the central atom and the number of lone pairs of electrons attached to the central atom. Note that steric numbers are determined by first drawing the Lewis dot structure of the compound or ion. ...
... The steric number is determined by counting the number of bonded groups attached to the central atom and the number of lone pairs of electrons attached to the central atom. Note that steric numbers are determined by first drawing the Lewis dot structure of the compound or ion. ...
Chapter 4 - Jenkins Independent Schools
... Earth’s crust contains less than one percent carbon, yet all living things on Earth are made of carbon-containing compounds. Carbon’s ability to bond easily and form compounds is the basis of life on Earth. A carbon atom has four electrons in its outer energy level, so it can form covalent bonds wit ...
... Earth’s crust contains less than one percent carbon, yet all living things on Earth are made of carbon-containing compounds. Carbon’s ability to bond easily and form compounds is the basis of life on Earth. A carbon atom has four electrons in its outer energy level, so it can form covalent bonds wit ...
Chapter 8 - Inorganic carbon chemistry
... behind (Figure 8.13). These white deposits are calcium or magnesium sulphates and/or calcium carbonate. Calcium carbonate causes the 'furring' in kettles that occurs in hard water areas (Figure 8.14a). This furring may be removed by the addition of a dilute acid: hydrogen ions + carbonate ion carb ...
... behind (Figure 8.13). These white deposits are calcium or magnesium sulphates and/or calcium carbonate. Calcium carbonate causes the 'furring' in kettles that occurs in hard water areas (Figure 8.14a). This furring may be removed by the addition of a dilute acid: hydrogen ions + carbonate ion carb ...
Slide 1
... 2. The multiple bond of an alkene produces geometric isomers (cis and trans) a. Cis and trans isomers of alkenes behave as distinct compounds with different chemical and physical properties 3. The hydrogen atom of a terminal alkyne can be removed as H+, forming an acetylide ion (R–CC–) a. Acetylide ...
... 2. The multiple bond of an alkene produces geometric isomers (cis and trans) a. Cis and trans isomers of alkenes behave as distinct compounds with different chemical and physical properties 3. The hydrogen atom of a terminal alkyne can be removed as H+, forming an acetylide ion (R–CC–) a. Acetylide ...
Class 11 Class 12 The p- Block Element • Group13 (B to Tl
... Diamond :In diamond each carbon atom undergas SP3hybridisation. Each carbon is tetrahedrally linked to four other carbon atoms. Graphite :In graphite, carbon is SP2-hyberdized graphite has a two-dimensional sheet like structure consisting of a number of hexagonal rings fused together. Graphite condu ...
... Diamond :In diamond each carbon atom undergas SP3hybridisation. Each carbon is tetrahedrally linked to four other carbon atoms. Graphite :In graphite, carbon is SP2-hyberdized graphite has a two-dimensional sheet like structure consisting of a number of hexagonal rings fused together. Graphite condu ...
BIOL 157 – BIOLOGICAL CHEMISTRY I Lecture 1 Elemental
... survive for any length of time. Another helium nucleus can fuse with Be to form Carbon (12C). Another fusion with helium forms oxygen (16O). The largest atom that can form from the nuclear fusion reactions is iron (56Fe). Those atoms that are larger than Fe are formed when the neutrons resulting fro ...
... survive for any length of time. Another helium nucleus can fuse with Be to form Carbon (12C). Another fusion with helium forms oxygen (16O). The largest atom that can form from the nuclear fusion reactions is iron (56Fe). Those atoms that are larger than Fe are formed when the neutrons resulting fro ...
qp31 - Smart Edu Hub
... Draw the structural formula of a man-made polymer with the same linkage. ...
... Draw the structural formula of a man-made polymer with the same linkage. ...
PDF Full-text
... of metallic and ceramic materials under severe sliding conditions. ILs have also been used for the reduction of friction coefficients and wear rates of thermoplastic polymers and epoxy resins. The first molten salt which was in the liquid state at room temperature, ethylammonium nitrate ([C2H5NH3]NO ...
... of metallic and ceramic materials under severe sliding conditions. ILs have also been used for the reduction of friction coefficients and wear rates of thermoplastic polymers and epoxy resins. The first molten salt which was in the liquid state at room temperature, ethylammonium nitrate ([C2H5NH3]NO ...
Organic and Bio-Molecular Chemistry
... two linkages; nitrogen, boron and aluminum are trivalent, they can establish three linkages with different atoms. Therefore silicon and carbon, the two abundant tetravalent elements, are the most efficient scaffolds to build up tridimensional molecular structures. There is however an important diffe ...
... two linkages; nitrogen, boron and aluminum are trivalent, they can establish three linkages with different atoms. Therefore silicon and carbon, the two abundant tetravalent elements, are the most efficient scaffolds to build up tridimensional molecular structures. There is however an important diffe ...
Slide 1
... 2. The multiple bond of an alkene produces geometric isomers (cis and trans) a. Cis and trans isomers of alkenes behave as distinct compounds with different chemical and physical properties 3. The hydrogen atom of a terminal alkyne can be removed as H+, forming an acetylide ion (R–CC–) a. Acetylide ...
... 2. The multiple bond of an alkene produces geometric isomers (cis and trans) a. Cis and trans isomers of alkenes behave as distinct compounds with different chemical and physical properties 3. The hydrogen atom of a terminal alkyne can be removed as H+, forming an acetylide ion (R–CC–) a. Acetylide ...
Adsorption of cesium, thallium, strontium and cobalt radionuclides
... or nitric acid. The vials were kept in thermostat shaker bath at 298 K. After different periods of time the radioactivity of the solution was measured. For radioactivity measurements, the aliquots (0.05 cm3 ) were first dried on planchets under an infrared lamp and then counted under identical geome ...
... or nitric acid. The vials were kept in thermostat shaker bath at 298 K. After different periods of time the radioactivity of the solution was measured. For radioactivity measurements, the aliquots (0.05 cm3 ) were first dried on planchets under an infrared lamp and then counted under identical geome ...
eBook AQA GCSE Chemistry Unit C2 Part 1
... mixture, and can help us to identify the components. ...
... mixture, and can help us to identify the components. ...
stoich practice problems sp11
... 11. How many grams of iron are needed to react with 31.0 L of chlorine gas at STP to produce iron(III) chloride? 12. When 9.34 g of zinc react with excess hydrochloric acid how many grams of zinc chloride will be produced? 13. How many liters of oxygen gas at STP are required to react with 65.3 g of ...
... 11. How many grams of iron are needed to react with 31.0 L of chlorine gas at STP to produce iron(III) chloride? 12. When 9.34 g of zinc react with excess hydrochloric acid how many grams of zinc chloride will be produced? 13. How many liters of oxygen gas at STP are required to react with 65.3 g of ...
Chapter 10. Chemical Nomenclature
... reactive behavior, acids, bases and salts. Acids react with the bases in a process called ‘‘neutralization’’ to produce salts. In a sense acids and bases are opposites. This reflects their composition. For example, oxygen reacts with most elements to produce oxide compounds. The oxides of non-metal ...
... reactive behavior, acids, bases and salts. Acids react with the bases in a process called ‘‘neutralization’’ to produce salts. In a sense acids and bases are opposites. This reflects their composition. For example, oxygen reacts with most elements to produce oxide compounds. The oxides of non-metal ...
Orbital hybridisation From Wikipedia, the free encyclopedia Jump to
... Chemist Linus Pauling first developed the hybridisation theory in order to explain the structure of molecules such as methane (CH4).[2] This concept was developed for such simple chemical systems, but the approach was later applied more widely, and today it is considered an effective heuristic for r ...
... Chemist Linus Pauling first developed the hybridisation theory in order to explain the structure of molecules such as methane (CH4).[2] This concept was developed for such simple chemical systems, but the approach was later applied more widely, and today it is considered an effective heuristic for r ...
carbon compounds - Badhan Education
... absence of oxygen. The fibres decompose to produce carbon fibres. Carbon fibres are used in spacecrafts and for making sports etc. Plastic. Plastic are materials which can moulded or set into a desired shape. Polyamides, polyesters and polythene (polyethylene) are common plastics. Thermoplatics. The ...
... absence of oxygen. The fibres decompose to produce carbon fibres. Carbon fibres are used in spacecrafts and for making sports etc. Plastic. Plastic are materials which can moulded or set into a desired shape. Polyamides, polyesters and polythene (polyethylene) are common plastics. Thermoplatics. The ...
Allotropes of carbon

Carbon is capable of forming many allotropes due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades many more allotropes and forms of carbon have been discovered and researched including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperature or extreme pressures.