Chemistry in Focus: Tiny Thermometers
... It turns out that the tiny thermometers were produced by accident. The Japanese scientists were actually trying to make tiny (nanoscale) gallium nitride wires. However, when they examined the results of their experiment, they discovered tiny tubes of carbon atoms that were filled with elemental gall ...
... It turns out that the tiny thermometers were produced by accident. The Japanese scientists were actually trying to make tiny (nanoscale) gallium nitride wires. However, when they examined the results of their experiment, they discovered tiny tubes of carbon atoms that were filled with elemental gall ...
Chapter 2 PROPERTIES OF FLUIDS
... a pure substance changes phase at a given pressure. • Saturation pressure Psat: The pressure at which a pure substance changes phase at a given temperature. • Vapor pressure (Pv): The pressure exerted by its vapor in phase equilibrium with its liquid at a given temperature. It is identical to the sa ...
... a pure substance changes phase at a given pressure. • Saturation pressure Psat: The pressure at which a pure substance changes phase at a given temperature. • Vapor pressure (Pv): The pressure exerted by its vapor in phase equilibrium with its liquid at a given temperature. It is identical to the sa ...
Vocabulary of Thermodynamics
... as lines separating the single-phase regions (labeled solid, liquid and vapor). The triple line is now shown in end view as a point. The second figure shows the P-v-T surface viewed perpendicular to the pressure and specific volume axes. Lines of constant temperature (isotherms) are shown. Notice th ...
... as lines separating the single-phase regions (labeled solid, liquid and vapor). The triple line is now shown in end view as a point. The second figure shows the P-v-T surface viewed perpendicular to the pressure and specific volume axes. Lines of constant temperature (isotherms) are shown. Notice th ...
More Thermodynamics
... temperature of a real gas will always decrease upon undergoing a free expansion. How much the temperature decreases depends upon the state point and the parameter a. Molecules having strong attractive interactions (a large a) should show the largest temperature decrease upon expansion. We can unders ...
... temperature of a real gas will always decrease upon undergoing a free expansion. How much the temperature decreases depends upon the state point and the parameter a. Molecules having strong attractive interactions (a large a) should show the largest temperature decrease upon expansion. We can unders ...
Units of Measurement - Karen Timberlake`s chemistry
... Is a decimal system based on 10 Used in most of the world Used by scientists and hospitals ...
... Is a decimal system based on 10 Used in most of the world Used by scientists and hospitals ...
The Zeroth Law of Thermodynamics
... "temperature" exists. Because heat flow is often associated with temperature gradients it is of course important to establish the existence of this property in order to understand heat flow. Because of how it is measured "temperature" was assumed to be a concrete and measureable property, even thoug ...
... "temperature" exists. Because heat flow is often associated with temperature gradients it is of course important to establish the existence of this property in order to understand heat flow. Because of how it is measured "temperature" was assumed to be a concrete and measureable property, even thoug ...
National Diploma in Engineering Mechanical Principles for
... To gain a distinction for this assignment you need to solve the problem using two alternative approaches, one being D`Alembert`s principle, and the other being the principle of the conservation of energy. The principle of conservation of energy equates work done in a system to changes in potential e ...
... To gain a distinction for this assignment you need to solve the problem using two alternative approaches, one being D`Alembert`s principle, and the other being the principle of the conservation of energy. The principle of conservation of energy equates work done in a system to changes in potential e ...
Thermodynamics
... a) Reversible expansion to 10 L under 2 atm b) Adiabatic free expansion c) Isothermal, reversible, compression to 2 L d) Isobaric, isothermal, irreversible expansion to 0.015 m3 under 2 atm e) Isothermal free expansion ...
... a) Reversible expansion to 10 L under 2 atm b) Adiabatic free expansion c) Isothermal, reversible, compression to 2 L d) Isobaric, isothermal, irreversible expansion to 0.015 m3 under 2 atm e) Isothermal free expansion ...
Notes on the First Law of Thermodynamics Chemistry CHEM 213W
... series of experiments are performed whereby weights are moved to pan from platforms at various heights in the surroundings. In doing so, the system (the spring and pan) move from state I to II. How much work is performed in each of the cases (a)−(c) (assume that there is a total 1cm elongation of th ...
... series of experiments are performed whereby weights are moved to pan from platforms at various heights in the surroundings. In doing so, the system (the spring and pan) move from state I to II. How much work is performed in each of the cases (a)−(c) (assume that there is a total 1cm elongation of th ...
Heat
... Samples with the same number of moles have the same number of atoms, and we conclude that the heat energy required per atom to raise the temperature of a solid by a given amount seems with a few exceptions to be about the same for all ...
... Samples with the same number of moles have the same number of atoms, and we conclude that the heat energy required per atom to raise the temperature of a solid by a given amount seems with a few exceptions to be about the same for all ...
CRYOGENICS
... The following methods are involved to produce the low temperature in cryogenics: Heat conduction: It is a relatively simple concept to understand. When two bodies are in contact, heat flows from the body with the higher temperature to the body with a lower temperature. Conduction can occur between a ...
... The following methods are involved to produce the low temperature in cryogenics: Heat conduction: It is a relatively simple concept to understand. When two bodies are in contact, heat flows from the body with the higher temperature to the body with a lower temperature. Conduction can occur between a ...
v = Y
... K Carnot = 1 − T H − TC “No engine can be more efficient than a Carnot Engine operating between the two ...
... K Carnot = 1 − T H − TC “No engine can be more efficient than a Carnot Engine operating between the two ...
N-Body Dynamics of Strongly- Coupled (Nonideal) Plasmas
... is based on the two main assumptions: (1) Effective potential for the motion of a quasitrapped electron in the field of a nearby ion is ...
... is based on the two main assumptions: (1) Effective potential for the motion of a quasitrapped electron in the field of a nearby ion is ...
Joule-Thomson Expansion
... The porous plate will allow a gas to go through it, but only slowly. It acts as a throttle. On each side of the plate there is a piston that fits the tube tightly. Each piston can (in principle) be pushed up against the porous plate. The tube itself is insulated so that no heat can enter or leave th ...
... The porous plate will allow a gas to go through it, but only slowly. It acts as a throttle. On each side of the plate there is a piston that fits the tube tightly. Each piston can (in principle) be pushed up against the porous plate. The tube itself is insulated so that no heat can enter or leave th ...
Model Question Paper – 1
... The internal energy of a certain substance is given by u = 3.56 Pv + 84 where u is in kJ/kg; P is in Pa; and v is in m3 / kg. A system of gas of 3 kg expands from initial pressure of 500 kPa and volume of 0.22 m3 to a final pressure of 100 kPa in a process described by PV1.2 = constant. a) If expans ...
... The internal energy of a certain substance is given by u = 3.56 Pv + 84 where u is in kJ/kg; P is in Pa; and v is in m3 / kg. A system of gas of 3 kg expands from initial pressure of 500 kPa and volume of 0.22 m3 to a final pressure of 100 kPa in a process described by PV1.2 = constant. a) If expans ...
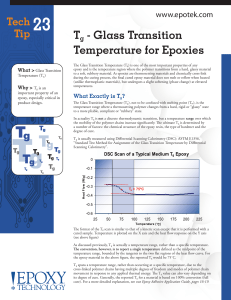
Tg - Glass Transition Temperature for Epoxies
... As discussed previously, Tg is actually a temperature range, rather than a specific temperature. The convention, however, is to report a single temperature defined as the midpoint of the temperature range, bounded by the tangents to the two flat regions of the heat flow curve. For the epoxy material ...
... As discussed previously, Tg is actually a temperature range, rather than a specific temperature. The convention, however, is to report a single temperature defined as the midpoint of the temperature range, bounded by the tangents to the two flat regions of the heat flow curve. For the epoxy material ...
ln2_storage_pre
... On the other hand, the gas cylinders are probably at room temperature. This is way above the critical temperature for both fluids, so you will not get a liquid no matter how much pressure you put on it. The gases in the cylinders are supercritical fluids, though when you get that far above the criti ...
... On the other hand, the gas cylinders are probably at room temperature. This is way above the critical temperature for both fluids, so you will not get a liquid no matter how much pressure you put on it. The gases in the cylinders are supercritical fluids, though when you get that far above the criti ...