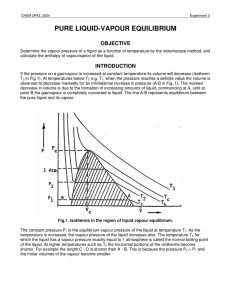
pure liquid-vapour equilibrium - Theoretical and Computational
... approximation Vvap = Vv. The molar volume of the liquid can be neglected compared to that of the vapour. Defining the compressibility factor of the vapour as ...
... approximation Vvap = Vv. The molar volume of the liquid can be neglected compared to that of the vapour. Defining the compressibility factor of the vapour as ...
thermodynamics - La Salle High School
... If the entropy of each element in its most state is taken as zero at the absolute zero of temperature, every substance has a positive entropy. But at 0K, the entropy of substance may equals to 0, and does ...
... If the entropy of each element in its most state is taken as zero at the absolute zero of temperature, every substance has a positive entropy. But at 0K, the entropy of substance may equals to 0, and does ...
Lecture Notes 27
... It was found experimentally that if 1 mole of any gas is placed in containers that have the same volume V and are kept at the same temperature T , approximately all have the same pressure p. The small differences in pressure disappear if lower gas densities are used. Further experiments showed that ...
... It was found experimentally that if 1 mole of any gas is placed in containers that have the same volume V and are kept at the same temperature T , approximately all have the same pressure p. The small differences in pressure disappear if lower gas densities are used. Further experiments showed that ...
Document
... semiconductor materials. In the cooling mode, direct current is allowed to passes through n and p junction of a semiconductor material. The temperature, denoted as TC (Cold Temperature) of the interconnecting conductor is decreased while heat is absorbed from the environment. This heat absorption fr ...
... semiconductor materials. In the cooling mode, direct current is allowed to passes through n and p junction of a semiconductor material. The temperature, denoted as TC (Cold Temperature) of the interconnecting conductor is decreased while heat is absorbed from the environment. This heat absorption fr ...
LECTURE 7 General Relations for a Homogeneous Substance For
... Notice that the conjugate variables are always paired up. The sign changes when one changes the independent variable compared to the fundamental relation (42). Phase Transitions and the Clausius–Clapeyron Equation Let me try to give some idea of why these thermodynamic functions are useful. We know ...
... Notice that the conjugate variables are always paired up. The sign changes when one changes the independent variable compared to the fundamental relation (42). Phase Transitions and the Clausius–Clapeyron Equation Let me try to give some idea of why these thermodynamic functions are useful. We know ...
1.Concept of the glassy state: 1-1 Definition of glassy state: Glassy
... Glassy state: is a state of matter that combines the rigidity of crystals with the random molecular structure of liquids. Glass is defined in ASTM as 'an inorganic product of fusion which has been cooled to a rigid condition without crystallization'. More specifically, the term is used to relate to ...
... Glassy state: is a state of matter that combines the rigidity of crystals with the random molecular structure of liquids. Glass is defined in ASTM as 'an inorganic product of fusion which has been cooled to a rigid condition without crystallization'. More specifically, the term is used to relate to ...
Class notes
... substituting for V = nRT/pi and integrating from a partial pressure of a compound defined as pi0 to pi ig = RT ln pi/pi0 ...
... substituting for V = nRT/pi and integrating from a partial pressure of a compound defined as pi0 to pi ig = RT ln pi/pi0 ...
2.2.35. Osmolality
... The observations made in thermomicroscopy allow the nature of the phenomena detected using thermogravimetry the mixing device is programmed to operate at a temperature below that expected through cryoscopic depression to and differential thermal analysis to be clearly identified. prevent supercoolin ...
... The observations made in thermomicroscopy allow the nature of the phenomena detected using thermogravimetry the mixing device is programmed to operate at a temperature below that expected through cryoscopic depression to and differential thermal analysis to be clearly identified. prevent supercoolin ...
Thermodynamics
... with each other Allows for a definition of temperature (two objects have the same temperature when they are in thermal equilibirum) ...
... with each other Allows for a definition of temperature (two objects have the same temperature when they are in thermal equilibirum) ...
Oxide-ceramic products for high-temperature technology
... metals with high oxygen affinity, such as Ti und Zr, which can directly react with the ceramic base material. This property, disadvantageous as regards corrosion resistance, is today used to advantage in the manufacture of brazed, high-vacuum-tight products made of Al2O3 and ZrO2 ceramics and metal ...
... metals with high oxygen affinity, such as Ti und Zr, which can directly react with the ceramic base material. This property, disadvantageous as regards corrosion resistance, is today used to advantage in the manufacture of brazed, high-vacuum-tight products made of Al2O3 and ZrO2 ceramics and metal ...
For a full listing and description of the equipment in the Biomaterials
... It measures the texture and hardness of our biomaterials. It is able to measure the rheological characteristics of extremely soft materials such as lipid networks (e.g. butter) and the compressive and tensile strengths of hard plastic materials. It is also capable of precisely quantifying a wide var ...
... It measures the texture and hardness of our biomaterials. It is able to measure the rheological characteristics of extremely soft materials such as lipid networks (e.g. butter) and the compressive and tensile strengths of hard plastic materials. It is also capable of precisely quantifying a wide var ...
Real Gases
... As mentioned, this process can be inverted and we can solve for B'(T), C'(T), etc. in terms of B(T), C(T), etc. This gives: B'(T) = C'(T) = etc. Finally, real gases also differ from an Ideal gas in that if cooled or compressed, they will typically condense into a liquid. This behavior can be illustr ...
... As mentioned, this process can be inverted and we can solve for B'(T), C'(T), etc. in terms of B(T), C(T), etc. This gives: B'(T) = C'(T) = etc. Finally, real gases also differ from an Ideal gas in that if cooled or compressed, they will typically condense into a liquid. This behavior can be illustr ...
Introduction to Physical Chemistry – Lecture 7
... (∂U/∂V )T , measures the dependence of a system’s internal energy on volume, assuming the temperature is held constant. Intuitively, then, this quantity should provide a measure of the strength of the interparticle interactions in a material. The reason for this is that changing V essentially corres ...
... (∂U/∂V )T , measures the dependence of a system’s internal energy on volume, assuming the temperature is held constant. Intuitively, then, this quantity should provide a measure of the strength of the interparticle interactions in a material. The reason for this is that changing V essentially corres ...
Study of Thermal Resistance Measurement Techniques
... Sometimes different thermocouple systems differed at certain temperatures but agree at other temperatures. To choose a thermocouple system, it is examined versus a precision thermometer. c) Current Sources: Making a perfect Current Source is difficult and can lead to oscillations due to the small lo ...
... Sometimes different thermocouple systems differed at certain temperatures but agree at other temperatures. To choose a thermocouple system, it is examined versus a precision thermometer. c) Current Sources: Making a perfect Current Source is difficult and can lead to oscillations due to the small lo ...
Comparison of entropy difference in the cooling process
... We saw that water has the highest cooling potential as a working fluid for evaporation. For desorption, H2 desorbing from UHx has the highest potential. In the case of isothermal expansion, a compression ratio of 50 seems more than reasonable as an upper limit. Gd5 Ge2 Si2 experienced the highest ent ...
... We saw that water has the highest cooling potential as a working fluid for evaporation. For desorption, H2 desorbing from UHx has the highest potential. In the case of isothermal expansion, a compression ratio of 50 seems more than reasonable as an upper limit. Gd5 Ge2 Si2 experienced the highest ent ...
Chemistry 3510: Physical Chemistry Midterm Exam 1 19 February 2007 Name:
... starting from the total differential of the internal energy and first law (with only pV work). ...
... starting from the total differential of the internal energy and first law (with only pV work). ...
AOS 100: Weather and Climate
... 0% RH, and is about 9.8ºC/km - The moist adiabatic lapse rate (MALR) is for a parcel that is saturated, and is close to 6.5ºC/km in the lower atmosphere - This difference is caused by the release of latent heat as water changes phase (like from a gas to a liquid), which warms the air ...
... 0% RH, and is about 9.8ºC/km - The moist adiabatic lapse rate (MALR) is for a parcel that is saturated, and is close to 6.5ºC/km in the lower atmosphere - This difference is caused by the release of latent heat as water changes phase (like from a gas to a liquid), which warms the air ...
Vapor Pressure of a Pure Liquid
... and enter the gas phase until the pressure of the vapor in the bulb reaches a definite value which is determined by the nature of the liquid and its temperature. This is called the vapor pressure of the liquid. In this experiment, the variation of vapor pressure with temperature will be measured and ...
... and enter the gas phase until the pressure of the vapor in the bulb reaches a definite value which is determined by the nature of the liquid and its temperature. This is called the vapor pressure of the liquid. In this experiment, the variation of vapor pressure with temperature will be measured and ...




















![Thermal properties Heat capacity C = ΔQ/ΔT = dQ/dT [J/deg] Heat](http://s1.studyres.com/store/data/015132718_1-30af002d7b96997c56f474559859d37b-300x300.png)
