* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture Notes 27
State of matter wikipedia , lookup
Thermal expansion wikipedia , lookup
Equipartition theorem wikipedia , lookup
Van der Waals equation wikipedia , lookup
Temperature wikipedia , lookup
Entropy in thermodynamics and information theory wikipedia , lookup
Internal energy wikipedia , lookup
Heat transfer physics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermodynamic system wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Equation of state wikipedia , lookup
History of thermodynamics wikipedia , lookup
Chapter
19:
The
Kine0c
Theory
of
Gases
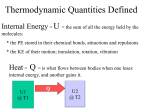
Thermodynamics = macroscopic picture
Gases micro -> macro picture
Avogadro’s
Number
One mole is the number of atoms in 12 g sample
of carbon-12
NA=6.02 x 1023 mol-1
So the number of moles n is given by
n=N/NA
C(12)—6 protrons, 6 neutrons and 6 electrons
12 atomic units of mass assuming mP=mn
Another way to do this is to
know the mass of one
molecule: then
N=
M sample
m mole−mass
NA
Ideal
Gas
Law
Ideal Gases, Ideal Gas Law
It was found experimentally that if 1 mole of any gas is placed in containers that
have the same volume V and are kept at the same temperature T , approximately all
have the same pressure p. The small differences in pressure disappear if lower gas
densities are used. Further experiments showed that all low-density gases obey the
equation pV = nRT . Here R = 8.31 K/mol ⋅ K and is known as the "gas constant."
The equation itself is known as the "ideal gas law." The constant R can be expressed
as R = kN A . Here k is called the Boltzmann constant and is equal to 1.38 × 10-23 J/K.
If we substitute R as well as n =
N
in the ideal gas law we get the equivalent form:
NA
pV = NkT . Here N is the number of molecules in the gas.
The behavior of all real gases approaches that of an ideal gas at low enough densities.
= number
moles
Nenough
= number
of particles
Low densitiesn means
thatofthe
gas molecules
are far
apart
that they do not
interact with one another, but only
with the walls of the gasgas
container.
constant
Boltzmann Constant
pV = nRT
pV = NkBT
kB = 1.38×10-23 J/K
R = kBNA R = 8.315 J/(mol⋅K) = 0.0821 (L⋅atm)/(mol⋅K) = 1.99
calories/(mol⋅K) Work
Done
by
Isothermal
(ΔT
=
0)
Expansion/Compression
of
Ideal
Gas
On p-V graph, the green lines are isotherms…
… each green line corresponds to a system at a constant temperature.
From ideal gas law, this means that for a given isotherm:
⇒ p = (nRT )
pV = constant
Wby =
∫
Vi
Vf
f
i
i
V
nRT
dV
pdV = ∫
dV = nRT ∫
V V
V V
For expansion, we have: Vf > Vi
€
For compression, we have: Vf < Vi
Wby > 0
€
Relates p and V
Vf
⇒ Wby,isothermal = nRT ln
ΔT =0
Vi
p
= nRT ln i
pf
€ is then: The work done by the gas
Vf
1
V
Wby < 0
Checkpoint 1: An ideal gas has an initial pressure of 3
pressure units and an initial volume of 4 volume units. The
table gives the final pressure and volume of the gas in five
processes. Which processes start and end on the same
isotherm?
– a
• p
12
• V 1
b
c
d
e
6
2
5
7
4
3
1
12
pV=nRT in this case T is a constant so pV=C=12
Sample problem #19-2
One mole of oxygen expands at a constant temperature T
of 310 K from an initial volume Vi of 12 L to a final volume
Vf of 19 L. How much work is done by the gas during the
expansion.
We calculated W for isothermal
W=nRT ln (Vf /VI)
W= (1 mole)(8.31J/mole K)(310K) ln(19/12)
W=1180 J
Work Done by Isobaric (Δp = 0) Expansion of an Ideal Gas
Vf
Wby =
∫ pdV
Vi
⇒ Wby,isobaric = pΔV
ΔP=0
= nRΔT
€
€
Adiaba0c
Expansion
of
an
ideal
gas
(Q
=
0)
Because a gas is thermally insulated, or expansion/compression happens suddenly ⇒ adiabatic Remember “Adiabatic means Q = 0” or, by 1st Law of Thermo ⇒ ΔEint = -Wby
In this case
pVγ = constant
where
γ = cp/cV = (R+ cV )/cV example: monatomic gas γ = 5/3 γ
1 1
γ
2
p V = p2V
T1V1γ −1 = T2V2γ −1
Compare with Isothermal Expansion (ΔT = 0)
€
T1 = T2 ⇔
[ p1V1 = p2V2 ] isothermal
Pressure, Temperature, & RMS speed
Assume the collision of the gas molecule with the wall is
elastic then the
:
Δpx = (−mvx ) − (mvx ) = -2mvx
The molecule travels to the back wall, collides and comes back. The time it takes
is 2L/vx.
2
The
is F/A
n
p=
Δp 2mvx mvx
=
=
Δt 2l / vx
L
2
xi
n
Fx
1
mv
m
= 2∑
= 3 ∑ vxi2
A L i =1 L
L i =1
2
x avg
If we calculated the average velocity
and use the fact that the number in the sum
n
is nNA then:
2
2
nM (v )
nmN A 2
p=
(vx )avg =
3
L
V
2
2
2
2
v
=
v
+
v
+
v
x
y
z
2
nM (v 2 )avg nMvrms
€
2
=
=
v
3V
3V
vx2 =
3
(v )
x
avg
=
∑v
xi
nN A
i=1
2
(v )avg = vrms
RMS = Root-Mean-Square
RMS
Speeds
We have
pressure
p=
For ideal gas
€
vrms
3pV
=
nM
vrms =
2
nMv rms
3V
pV = nRT
1/2
3nRT
=
nM
1/2
3RT
M
The RMS velocity depends on:
Molar mass & Temperature
Problem #19-3: Here are five numbers: 5, 11, 32,
67, and 89. (a) What is the average value navg?
(b) What is the rms value nrms of the numbers?
(a)
navg
5 + 11 + 32 + 67 + 89
=
= 40.8
5
(b)
(n 2 )avg
1 n 2 5 2 + 112 + 32 2 + 67 2 + 89 2
= ∑ ni =
= 2714.41
n i =1
5
(n 2 )avg = 52.1
n avg ≠
(n 2 )avg = n rms
Problem #19-21: (a) Compute the rms speed of a nitrogen
molecule at 20.0 0C (each N atom has 7 protons and 7
neutrons). At what temperatures will the rms speed be (b) half
that value and (c) twice that value?
3RT
v rms =
M
Remember to use: T = 20 °C + 273 = 293 K
€
If
we
know
the
“average”
speed
of
par0cles,
we
then
know
the
kine0c
energy,
1 3RT
1
2
KE = m (vrms ) = m
2 M
2
What
is
the
rela0onship
between
transla0onal
€
kine0c
energy
&
internal
energy?
Transla0onal
Kine0c
Energy
&
Internal
energy
1 3RT
1
2
KE = m (vrms ) = m
2 M
2
3
KE = kB T
2
€
M = mN A
kB= R N A
The
€ KE of all ideal gas molecules
depends only on the temperature
€(not mass!)
€
Monoatomic ideal gas : He, Ar, Ne, Kr… (no potential energies)
Eint, monotonic = N A
(
3
2
)
kB T = 32 nRT The internal energy
ΔE int,monotonic = 32 nR(ΔT )
€
of an ideal gas depends only on the temperature
Problem #19-26: What is the average translational
kinetic energy of 1 mole nitrogen molecules at 1600 K?
Molar
Specific
Heat
at
Constant
Volume:
Monatomic
Ideal
Gas
Molar Specific Heat at Constant Volume (Wby=0)
Q = ncV ΔT
&
Wby = 0
ΔEint = Q = ncV ΔT
€
ΔE int = n( 32 R)ΔT = n(CV )ΔT = Q
€
cV , monatomic =
€
€
3
R =12.5 ⋅ J/mol ⋅ K
2
Molar
Specific
Heat:
Monatomic
ideal
gas
Molar Specific Heat at Constant Volume (Wby=0)
ΔEint = Q = ncV ΔT
€
cV , monatomic =
3
R =12.5 ⋅ J/mol ⋅ K
2
Molar Specific Heat at Constant Pressure (Wby=pΔV)
€
ΔEint = Q − Wby = nc p ΔT − pΔV
ncV ΔT = nc p ΔT − nRΔT
cV = c p − R
€
€
€
€
c p, monatomic =
or
c p = cV + R
5
R = 20.8 ⋅ J/mol ⋅ K
2
Remember
special
cases…
Adiabatic expansion/contraction - NO TRANSFER OF ENERGY AS HEAT
Q = 0
[ΔE int = −W ] adiabatic
Constant-volume processes (isochoric)-
NO WORK IS DONE
ΔE int = Q
W = 0
Wby =
∫ pdV
= area under p − V graph
∫ pdV = 0
Vi
QΔV = 0 = nCV ΔT
Constant-pressure processes €
Wby =
V f =Vi
ΔE int = Q − pΔV
€
CV = C P − R
3)Cyclical process (closed cycle)
€
net area in p-V curve is Q
€
€
V f =Vi
Wby =
∫ pdV = pΔV
Vi
QΔP= 0 = nC P ΔT
ΔEint,closed cycle =0
ΔE int = 0 ⇒ Q = Wby
Chapter 20: Entropy and the Second law of thermodynamics
0th law
Thermal Equilibrium: A = B & B = C then A = C
1st law
Q = ncΔT
Q → 0 as ΔT → 0 Conservation of energy: ΔEint = Q - Wby= Q + Won
Change in
Internal energy = heat added minus work done by
Today:
2nd law
HEAT FLOWS NATURALLY FROM HOT OBJECT TO A
COLD OBJECT
Heat will NOT flow spontaneously from cold to hot
0 ≤ ΔS total
Hall
of
fame
Ludwig Boltzmann
(1844-1906)
Boltzmann constant kB =1.38×10-23 J/K
W = number of states
Irreversible
Processes
How
to
understand
this:
Entropy
How to describe a system:
P, T, V, Eint, and n
Entropy, S, like T,V, P, Eint, and n is state variable
How to define entropy? Easier to define Change of entropy during a process.
f
ΔS part = S f − S i =
∫
i
dQ
T
Temp in Kelvin
Units: [J/K]
where Q is energy transferred to or from a system during a process
[ Note: since T > 0, if Q is positive (negative) the ΔS is positive (negative) ] What
€ is a process? expansion, compression, temperature rise, add mass Reversible process
{processes can be done infinitely slowly to ensure thermal equilibrium}
The Second Law of Thermodynamics
The entropy of a closed system (no energy and no mass comes in and out) never decreases. It either stays constant (reversible process) or increases (irreversible process).
0 ≤ ΔS total
€
Entropy:
Ideal
Gas
Processes
1) For reversible process: ΔS cycle,rev = 0 =
∫
dQ
T
2) For isothermal process: €
ΔS isothermal
Q
=
T
3) In general for gas, using 1st law: dE int = dQ − dW
nCV €
dT dQ pdV
=
−
T
T
T
nRT
& p€=
V
ΔE int = 0
⇒ Q=W
Vf
W = nRT ln ⇒
Vi
⇒
ΔS isothermal
nRT dV
€ dQ
=
∫
∫ +
T
V
T
∫
Vf
= nR ln
Vi
nCV dT
T
Now integrate:
V f
T f
ΔS general, gas = S f − S i = nR ln + nC V ln
Ti
€ Vi
€
4) For adiabatic (reversible) adiabatic compression/expansion (Q=0): €
ΔS rev,adiabatic = 0
⇐
[
pV = nRT ]
Entropy is a State
Function
Checkpoint:
An
ideal
gas
has
a
temperature
T1
at
the
iniDal
state
i
shown
in
the
p‐V
diagram.
The
gas
has
a
higher
temperature
T2
at
the
final
states
a
and
b,
which
can
reach
along
the
paths
shown.
Is
the
entropy
change
along
the
path
to
state
a
larger
than,
smaller
than,
or
the
same
as
that
along
path
to
state
b?
From i to a:
From i to b:
T2
ΔS = nCV ln
T1
T2
Vb
ΔS = nCV ln + nR ln
T1
Va
Entropy:
Liquid/solid
processes
1)
ΔS phase change =
For phase changes:
Temperature = constant ∫
dQ
T
Qphase change
T
mL
=
T
=
2) For temperature changes: €
ΔS liquid / solid = S f − S i =
∫
mcdT
∫
T
Tf
= mc ln
Ti
=
€
dQ
T
Sample
Problem
#20‐2:
Two
idenDcal
copper
blocks
of
mass
m=1.5kg:
Block
L
is
at
TiL=
600C
and
block
R
is
at
TiR=200C.
The
blocks
are
in
a
thermally
insulated
box
and
are
separated
by
an
insulaDng
shuTer.
When
we
liV
the
shuTer,
the
blocks
come
to
equilibrium
with
Tf=400C.
What
is
the
entropy
of
this
irreversible
process?
Specific
heat
of
Cu
is
386
J/kg‐K.
The left block is initially at 600C, and comes to equilibrium with final
temperature 400C. Heat is transferred from the left block to the right.
f
dQ = mcdT
Tf
Tf
dQ
mcdT
ΔSL = ∫
=∫
= mc ln
= −35.86J / K
T
T
TiL
i
Til
Now set reservoir at 200C and put in contact with R. Raise the temperature
slowly to 400C.
ΔSR = 38.23J / K
ΔSrev = ΔSR + ΔSL = 2.4J / K
Sample
problem
20‐1:
Suppose
1.0
mol
of
nitrogen
gas
is
confined
to
the
leV
side
of
the
container
in
the
figure.
You
open
the
stop‐cock
and
the
volume
of
the
gas
doubles.
What
is
the
entropy
change
of
the
gas
for
this
irreversible
process?
Free Expansion so
ΔT = 0
Q = nRT ln
(
)
€
Vf
Q nRT ln V f / Vi )
ΔS = =
= nR ln
T
T
Vi
Vf
Vi
Put in numbers
ΔS = +5.76J / K