Chemistry of CHLORINE
... -Chlorine in the gas jar is poisonous/toxic. -Burning phosphorus produces poisonous/toxic phosphorus (III) chloride // phosphorus (V) chloride. -Ensure the reaction is not affected by air/oxygen from the atmosphere. (d) After the reaction is complete, 2cm3 of distilled water were added. The solution ...
... -Chlorine in the gas jar is poisonous/toxic. -Burning phosphorus produces poisonous/toxic phosphorus (III) chloride // phosphorus (V) chloride. -Ensure the reaction is not affected by air/oxygen from the atmosphere. (d) After the reaction is complete, 2cm3 of distilled water were added. The solution ...
Dr David`s Chemistry Test Answers
... 1. It means that reactions can proceed in both the forward and reverse directions. Altering the conditions of the reaction can change the direction of the reaction. 2. Many familiar inorganic reactions like the reaction of hydrochloric acid with sodium hydroxide are not reversible. Similarly, the re ...
... 1. It means that reactions can proceed in both the forward and reverse directions. Altering the conditions of the reaction can change the direction of the reaction. 2. Many familiar inorganic reactions like the reaction of hydrochloric acid with sodium hydroxide are not reversible. Similarly, the re ...
1.24 calculations and chemical reactions
... A 25.0 cm3 sample of this solution required 22.80 cm3 of 0.150 mol dm–3 aqueous NaOH for complete reaction. Calculate the relative molecular mass, Mr, of H2A 4.2) Sodium carbonate forms several hydrates of general formula Na2CO3.xH2O. A 2.98 g sample of one of these hydrates was dissolved in water a ...
... A 25.0 cm3 sample of this solution required 22.80 cm3 of 0.150 mol dm–3 aqueous NaOH for complete reaction. Calculate the relative molecular mass, Mr, of H2A 4.2) Sodium carbonate forms several hydrates of general formula Na2CO3.xH2O. A 2.98 g sample of one of these hydrates was dissolved in water a ...
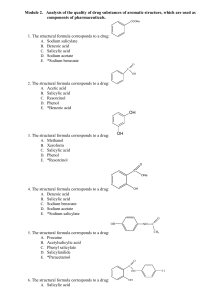
Module 2. Drug substances of aromatic structure
... D. Phenolphthalein E. *Solution of starch 81. The chemical name of xeroform: A. Oxybenzene B. Dihydroxybenzene C. 3-Methyl-5-methylphenol D. 5-Methyl-2-(methylethyl)phenol E. *Bismuth tribromophenol basic with bismuth oxide 82. For synthesis of thymol it is possible to use such initial substance: A. ...
... D. Phenolphthalein E. *Solution of starch 81. The chemical name of xeroform: A. Oxybenzene B. Dihydroxybenzene C. 3-Methyl-5-methylphenol D. 5-Methyl-2-(methylethyl)phenol E. *Bismuth tribromophenol basic with bismuth oxide 82. For synthesis of thymol it is possible to use such initial substance: A. ...
1.24 calculations and chemical reactions
... A 25.0 cm3 sample of this solution required 22.80 cm3 of 0.150 mol dm–3 aqueous NaOH for complete reaction. Calculate the relative molecular mass, Mr, of H2A 4.2) Sodium carbonate forms several hydrates of general formula Na2CO3.xH2O. A 2.98 g sample of one of these hydrates was dissolved in water a ...
... A 25.0 cm3 sample of this solution required 22.80 cm3 of 0.150 mol dm–3 aqueous NaOH for complete reaction. Calculate the relative molecular mass, Mr, of H2A 4.2) Sodium carbonate forms several hydrates of general formula Na2CO3.xH2O. A 2.98 g sample of one of these hydrates was dissolved in water a ...
1aUnit Two Handouts - Dunmore High School
... These materials may NOT be copied or redistributed in any way, except for individual class instruction. ...
... These materials may NOT be copied or redistributed in any way, except for individual class instruction. ...
Unit 7 Homework and Lab Packet
... 1. The principle ingredient of the self-defense agent mace is chloroacetophenone, C6H5COCH2Cl. This compound is prepared by chlorination of acetophenone, C6H5COCH3. What mass of chloroacetophenone would be produced by the reaction of 4.00 g of acetophenone with 10.4 g of chlorine? C6H5COCH3 + Cl2 ...
... 1. The principle ingredient of the self-defense agent mace is chloroacetophenone, C6H5COCH2Cl. This compound is prepared by chlorination of acetophenone, C6H5COCH3. What mass of chloroacetophenone would be produced by the reaction of 4.00 g of acetophenone with 10.4 g of chlorine? C6H5COCH3 + Cl2 ...
Complex Ion Equilibria - South Kingstown High School
... According to Le Chatelier’s principle, as C2O42ion is removed by the reaction with H3O+, more calcium oxalate dissolves. Therefore, you expect calcium oxalate to be more soluble in acidic solution (low pH) than in pure water. ...
... According to Le Chatelier’s principle, as C2O42ion is removed by the reaction with H3O+, more calcium oxalate dissolves. Therefore, you expect calcium oxalate to be more soluble in acidic solution (low pH) than in pure water. ...
practical identification of organic compounds.docx
... ether. This includes the lower members of the various homologous series (4 5 atoms in a normal chain) that contain oxygen and / or nitrogen in their structures : they are soluble in water because of their low carbon content. If the compound is soluble in both water and in ether, it would also be sol ...
... ether. This includes the lower members of the various homologous series (4 5 atoms in a normal chain) that contain oxygen and / or nitrogen in their structures : they are soluble in water because of their low carbon content. If the compound is soluble in both water and in ether, it would also be sol ...
Mole
... relationships between the amounts of reactants used and products formed by a chemical reactions; it is based on the law of conservation of mass. ...
... relationships between the amounts of reactants used and products formed by a chemical reactions; it is based on the law of conservation of mass. ...
1. (a) Propan-1ol, C2H5CH2OH can be oxidised to propanoic acid
... concentrated sulphuric acid was added. The mixture was heated under reflux until no further reaction was detectable. The mixture was then cooled rapidly to room temperature and titrated with 1.00 mol dm–3 sodium hydroxide solution. 35.0 cm3 of the 1.00 mol dm–3 sodium hydroxide solution was required ...
... concentrated sulphuric acid was added. The mixture was heated under reflux until no further reaction was detectable. The mixture was then cooled rapidly to room temperature and titrated with 1.00 mol dm–3 sodium hydroxide solution. 35.0 cm3 of the 1.00 mol dm–3 sodium hydroxide solution was required ...
Group 1: The Alkali Metals
... Alkali metals are known for being some of the most reactive metals. This is due in part to their larger atomic radii and low ionization energies. They tend to donate their electrons in reactions and often have an oxidation state of +1. These metals are characterized as being extremely soft and silve ...
... Alkali metals are known for being some of the most reactive metals. This is due in part to their larger atomic radii and low ionization energies. They tend to donate their electrons in reactions and often have an oxidation state of +1. These metals are characterized as being extremely soft and silve ...
2014_S4_CHM_NORMAL (ALL)
... statement is a correct explanation of the first statement. Then select one option from A to D according to the following table: A. B. C. D. ...
... statement is a correct explanation of the first statement. Then select one option from A to D according to the following table: A. B. C. D. ...
CH5 Student Revision Guides pdf
... The use of sodium thiosulfate(VI) in volumetric analysis Aqueous sodium thiosulfate(VI) is oxidised by aqueous iodine. The concentration of oxidising agents can be determined by reaction with excess aqueous iodide ions, and then titrating the iodine released with aqueous sodium thiosulfate. The aque ...
... The use of sodium thiosulfate(VI) in volumetric analysis Aqueous sodium thiosulfate(VI) is oxidised by aqueous iodine. The concentration of oxidising agents can be determined by reaction with excess aqueous iodide ions, and then titrating the iodine released with aqueous sodium thiosulfate. The aque ...
STOICHIOMETRY REVIEW WORKSHEET
... (d) How much oxygen was used up in moles? (e) How much oxygen was used up in grams? 2) Using the following equation: NaOH + ...
... (d) How much oxygen was used up in moles? (e) How much oxygen was used up in grams? 2) Using the following equation: NaOH + ...
advanced chemistry may 2011 marking scheme
... aqueous lead(II) nitrate in a small test tube several drops of aqueous iron(II) sulfate followed by about 1 mL of concentrated sulfuric acid which is allowed to form a separate bottom layer. ...
... aqueous lead(II) nitrate in a small test tube several drops of aqueous iron(II) sulfate followed by about 1 mL of concentrated sulfuric acid which is allowed to form a separate bottom layer. ...
Chemistry Test Ch 11 Stoichiometry
... B. In order to produce 6.52 L of NH3 how many liters of nitrogen are needed? C. If 2.35 x 1024 molecules of NH3 is formed how many grams of hydrogen was used? 2. Use the following equation answer these questions: Mg + 2 HNO3 Mg(NO3)2 + H2 A. How many grams of magnesium is need to react with 6.28 g ...
... B. In order to produce 6.52 L of NH3 how many liters of nitrogen are needed? C. If 2.35 x 1024 molecules of NH3 is formed how many grams of hydrogen was used? 2. Use the following equation answer these questions: Mg + 2 HNO3 Mg(NO3)2 + H2 A. How many grams of magnesium is need to react with 6.28 g ...
avogadro exam 1994 - University of Waterloo
... 14. What volume of argon gas at 100 kPa and 25°C must be added to a 1.00-Litre glass flask containing nitrogen gas at 70 kPa and 25°C to give a mixture of gases having a total pressure of 210 kPa at 25°C? ...
... 14. What volume of argon gas at 100 kPa and 25°C must be added to a 1.00-Litre glass flask containing nitrogen gas at 70 kPa and 25°C to give a mixture of gases having a total pressure of 210 kPa at 25°C? ...
aq - HCC Learning Web
... • Formulas and symbols are used to describe a chemical reaction as a chemical equation A+BC+D • A and B are reactants and C and D are products • Physical state is abbreviated by either (s), (l), (g), or (aq) • A catalyst is a substance that speeds up a reaction without being consumed or permanently ...
... • Formulas and symbols are used to describe a chemical reaction as a chemical equation A+BC+D • A and B are reactants and C and D are products • Physical state is abbreviated by either (s), (l), (g), or (aq) • A catalyst is a substance that speeds up a reaction without being consumed or permanently ...
Chemistry Tests Questions
... 14. What important property increases as the vertical arrangement of the noble (inert) gases is descended? 15. What is the family name of the group I elements? 16. Are the group one elements soft or hard? 17. How can you prevent the corrosion of sodium? 18. Which of the group I elements reacts most ...
... 14. What important property increases as the vertical arrangement of the noble (inert) gases is descended? 15. What is the family name of the group I elements? 16. Are the group one elements soft or hard? 17. How can you prevent the corrosion of sodium? 18. Which of the group I elements reacts most ...
A Few Things You Might Want To Know
... (distillation, crystallization, chromatography). Substances can be either elements or compounds. Compounds can be separated into elements by chemical changes (redox reactions). ...
... (distillation, crystallization, chromatography). Substances can be either elements or compounds. Compounds can be separated into elements by chemical changes (redox reactions). ...
PENTAL SODIUM (Thiopental sodium) and NORCURON
... In order to find interaction site of pental sodium and vecuronium bromide, firstly, the molecular geometry were evaluated in a point of minimal energy. After single point energy minimization (41.344 kcal /mol for pental sodium and 25.362 kcal/mol for vecuronium), three dimensional most probable stru ...
... In order to find interaction site of pental sodium and vecuronium bromide, firstly, the molecular geometry were evaluated in a point of minimal energy. After single point energy minimization (41.344 kcal /mol for pental sodium and 25.362 kcal/mol for vecuronium), three dimensional most probable stru ...
answer ch6 - Mr Khaled Nasr
... 67. What is the molar concentration of barium hydroxide, Ba(OH)2 in a solution formed by the reaction of 0.34g of barium with enough water to give 200.0mL of solution? (Ba = 137,O= 16, H = 1) ...
... 67. What is the molar concentration of barium hydroxide, Ba(OH)2 in a solution formed by the reaction of 0.34g of barium with enough water to give 200.0mL of solution? (Ba = 137,O= 16, H = 1) ...
Net Ionic Equations with Solubility R..
... 11. A precipitate is formed when solutions of trisodium phosphate and calcium chloride are mixed. 12. A solution of copper(II) sulfate is added to a solution of barium hydroxide. 13. Equal volumes of dilute equimolar solutions of sodium carbonate and hydrochloric acid are mixed. 14. Solid barium per ...
... 11. A precipitate is formed when solutions of trisodium phosphate and calcium chloride are mixed. 12. A solution of copper(II) sulfate is added to a solution of barium hydroxide. 13. Equal volumes of dilute equimolar solutions of sodium carbonate and hydrochloric acid are mixed. 14. Solid barium per ...
Mass - Mass Relationships
... How much silver carbonate is produced when 14.3 g of silver chloride reacts with excess sodium carbonate? What kind of reaction is this? Double displacement. Write the balanced equation. 2 AgCl + Na2CO3 Ag2CO3 + 2NaCl ...
... How much silver carbonate is produced when 14.3 g of silver chloride reacts with excess sodium carbonate? What kind of reaction is this? Double displacement. Write the balanced equation. 2 AgCl + Na2CO3 Ag2CO3 + 2NaCl ...
Sodium hydroxide

Sodium hydroxide (NaOH), also known as lye and caustic soda, is an inorganic compound. It is a white solid and highly caustic metallic base and alkali salt which is available in pellets, flakes, granules, and as prepared solutions at a number of different concentrations. Sodium hydroxide forms an approximately 50% (by weight) saturated solution with water.Sodium hydroxide is soluble in water, ethanol and methanol. This alkali is deliquescent and readily absorbs moisture and carbon dioxide in air.Sodium hydroxide is used in many industries, mostly as a strong chemical base in the manufacture of pulp and paper, textiles, drinking water, soaps and detergents and as a drain cleaner. Worldwide production in 2004 was approximately 60 million tonnes, while demand was 51 million tonnes.