Are You suprised ?
... 1. One of the main ingredients of pearls is calcium carbonate. If pearls are put in an acidic solution, they dissolve. CaCO3 + HCl CaCl2 + H2O + CO2 How many moles of CaCO3 can be dissolved in .0250 mol HCl? ...
... 1. One of the main ingredients of pearls is calcium carbonate. If pearls are put in an acidic solution, they dissolve. CaCO3 + HCl CaCl2 + H2O + CO2 How many moles of CaCO3 can be dissolved in .0250 mol HCl? ...
1. Cl2 + 2Br- ® 2Cl- + Br2 formulae correct for elements 1 correct
... are weak, do not allow they have weak forces / bonds) so little heat / energy is required before they can overcome forces / move freely / break out of solid structure / lattice (N.B. second point can be gained even if first is not) ...
... are weak, do not allow they have weak forces / bonds) so little heat / energy is required before they can overcome forces / move freely / break out of solid structure / lattice (N.B. second point can be gained even if first is not) ...
5 Things You Can Do to Reduce the Risk of Kidney Stones
... 1. Drink Plenty of Water: drinking extra water dilutes the substances in urine that lead to kidney stones. Drink enough fluids to pass 2 liters (2 liters is approx. 2 quarts) of urine every 24 hours. Your work environment may also determine how much you should have to drink: example, a person workin ...
... 1. Drink Plenty of Water: drinking extra water dilutes the substances in urine that lead to kidney stones. Drink enough fluids to pass 2 liters (2 liters is approx. 2 quarts) of urine every 24 hours. Your work environment may also determine how much you should have to drink: example, a person workin ...
1 - AlvarezHChem
... A solution is prepared by dissolving 10.8g ammonium sulfate in enough water to make 100.0mL of stock solution. A 10.00mL sample of this stock solution is added to 50.0mL of water. Calculate the concentration of ammonium ions and sulfate ions in the final solution. ...
... A solution is prepared by dissolving 10.8g ammonium sulfate in enough water to make 100.0mL of stock solution. A 10.00mL sample of this stock solution is added to 50.0mL of water. Calculate the concentration of ammonium ions and sulfate ions in the final solution. ...
Metals and non-metals III IMPORTANT POINTS Non-metals
... iii. Metal X forms the X2+ ion. Write a balanced equation, using X as the symbol for the metal, for the reaction that you would expect to take place when the hydroxide of X is heated in a dry test tube. ANSWERS 1. a. Magnesium, chromium and sodium are all metals, hence, they react with oxygen to for ...
... iii. Metal X forms the X2+ ion. Write a balanced equation, using X as the symbol for the metal, for the reaction that you would expect to take place when the hydroxide of X is heated in a dry test tube. ANSWERS 1. a. Magnesium, chromium and sodium are all metals, hence, they react with oxygen to for ...
C1a - Mr Corfe
... to the left of the periodic table or further down in the group (not including groups 3-8) TYPES OF REACTIONS PHYSICAL – changing of states EXOTHERMIC – gives out heat ENDOTHERMIC – take in heat from it surrounding THERMAL DECOMPOSITION – is a chemical reaction where a single compound breaks up into ...
... to the left of the periodic table or further down in the group (not including groups 3-8) TYPES OF REACTIONS PHYSICAL – changing of states EXOTHERMIC – gives out heat ENDOTHERMIC – take in heat from it surrounding THERMAL DECOMPOSITION – is a chemical reaction where a single compound breaks up into ...
wahideh chemistry eportfolio hw
... that English chemist Sir Humphry Davy (1778–1829) found a way to extract sodium from its compounds. Sodium metal itself has relatively few uses. It reacts with other substances easily, sometimes explosively. However, many sodium compounds have a variety of uses in industry, medicine, and everyday l ...
... that English chemist Sir Humphry Davy (1778–1829) found a way to extract sodium from its compounds. Sodium metal itself has relatively few uses. It reacts with other substances easily, sometimes explosively. However, many sodium compounds have a variety of uses in industry, medicine, and everyday l ...
Unit A Remediation Review
... 12. What are five clues that will allow you to conclude that a chemical change has occurred? 13. Describe what occurs in the following reaction types, the general equation and an example for each: a) Formation b) Decomposition c) Single Replacement d) Double Replacement e) Combustion 14. Write a bal ...
... 12. What are five clues that will allow you to conclude that a chemical change has occurred? 13. Describe what occurs in the following reaction types, the general equation and an example for each: a) Formation b) Decomposition c) Single Replacement d) Double Replacement e) Combustion 14. Write a bal ...
1.5.16(Chem) - mrcarlsonschemistryclass
... • Draw the funny way to remember cations and anions: ...
... • Draw the funny way to remember cations and anions: ...
Extra Credit Test Review
... 2. A _compound___ has a definite ratio of composition. 3. Chocolate milk could be described as which mixture? ___suspension___ (you must shake it up!) 4. A compound is separated only by ____chemical___ means. 5. Which of the following does NOT describe elements? a. all the particles are alike b. can ...
... 2. A _compound___ has a definite ratio of composition. 3. Chocolate milk could be described as which mixture? ___suspension___ (you must shake it up!) 4. A compound is separated only by ____chemical___ means. 5. Which of the following does NOT describe elements? a. all the particles are alike b. can ...
PDF (Size: 41K)
... Cyanogen, (CN)2, is a gas which is soluble in water to give the weak acid hydrocyanic acid, HCN (CN)2(g) + H2O(1) ...
... Cyanogen, (CN)2, is a gas which is soluble in water to give the weak acid hydrocyanic acid, HCN (CN)2(g) + H2O(1) ...
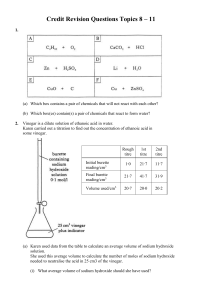
Credit Revision Questions Topics 8 – 11 1. (a) Which box contains a
... It reacts with cold water. ...
... It reacts with cold water. ...
Sodium hydroxide

Sodium hydroxide (NaOH), also known as lye and caustic soda, is an inorganic compound. It is a white solid and highly caustic metallic base and alkali salt which is available in pellets, flakes, granules, and as prepared solutions at a number of different concentrations. Sodium hydroxide forms an approximately 50% (by weight) saturated solution with water.Sodium hydroxide is soluble in water, ethanol and methanol. This alkali is deliquescent and readily absorbs moisture and carbon dioxide in air.Sodium hydroxide is used in many industries, mostly as a strong chemical base in the manufacture of pulp and paper, textiles, drinking water, soaps and detergents and as a drain cleaner. Worldwide production in 2004 was approximately 60 million tonnes, while demand was 51 million tonnes.