In-Class Exam - Fayetteville State University
... A) 2.64 B) 29.3 C) 26.4 D) 176 E) 50.0 26. One method for removal of metal ions from a solution is to convert the metal to its elemental form so it can be filtered out as a solid. Which metal can be used to remove aluminum ions from solution? A) cobalt B) copper C) lead D) zinc E) none of these 27. ...
... A) 2.64 B) 29.3 C) 26.4 D) 176 E) 50.0 26. One method for removal of metal ions from a solution is to convert the metal to its elemental form so it can be filtered out as a solid. Which metal can be used to remove aluminum ions from solution? A) cobalt B) copper C) lead D) zinc E) none of these 27. ...
IGCSE Revision document
... Sodium chloride NaCl is a ____________. There are __________ bonds between the two the same/ different numbers of electrons. elements _________ and _________. When these atoms bond one ____________ from the • Isotopes are atoms of the same element with ___________ atom is donated to the ____________ ...
... Sodium chloride NaCl is a ____________. There are __________ bonds between the two the same/ different numbers of electrons. elements _________ and _________. When these atoms bond one ____________ from the • Isotopes are atoms of the same element with ___________ atom is donated to the ____________ ...
Zuhmdahl (7th) Chapters 19, 20, 21 *Study Guide and Lecture Notes
... must read and outline each of the three chapters….shoot for a total of 10 sides of the page. Define any bold vocabulary terms and write out plenty of reaction examples. Use the examples below as practice. Precipitation Reaction Examples Solutions of calcium nitrate and sodium sulfate are mixed. Solu ...
... must read and outline each of the three chapters….shoot for a total of 10 sides of the page. Define any bold vocabulary terms and write out plenty of reaction examples. Use the examples below as practice. Precipitation Reaction Examples Solutions of calcium nitrate and sodium sulfate are mixed. Solu ...
The s-block - WordPress.com
... * The solutions are paramagnetic and on standing slowly liberate hydrogen resulting in the formation of amide. M+(am) + e− +NH3 (1)→MNH2(am)+½H2(g) ...
... * The solutions are paramagnetic and on standing slowly liberate hydrogen resulting in the formation of amide. M+(am) + e− +NH3 (1)→MNH2(am)+½H2(g) ...
AP Chemistry Summer Assignment
... For those students who have just taken Chemistry 1, much of the material in the summer packet will be familiar to you. For those students who have not taken Chemistry for a while the problems will help you rebuild a foundation in chemistry and insure all students are on a relatively even plane. It w ...
... For those students who have just taken Chemistry 1, much of the material in the summer packet will be familiar to you. For those students who have not taken Chemistry for a while the problems will help you rebuild a foundation in chemistry and insure all students are on a relatively even plane. It w ...
Name ______ Write formulas for the reactants and predicted
... Write formulas for the reactants and predicted products for the chemical reactions that follow. Assume that in all cases a reaction occurs. The equation must be balanced. Write all substances in their proper form –as ions if appropriate – and cancel any spectator ions. ...
... Write formulas for the reactants and predicted products for the chemical reactions that follow. Assume that in all cases a reaction occurs. The equation must be balanced. Write all substances in their proper form –as ions if appropriate – and cancel any spectator ions. ...
Precipitate Lab Report Power Point with Answers
... Temperature change, odor change, precipitate formation, irreversibility, color change, and new bubble formation are the evidence for a chemical reaction occuring. Not every time one of these changes is proof of a chemical reaction, but often they are. Sometimes chemical reactions can occur with no o ...
... Temperature change, odor change, precipitate formation, irreversibility, color change, and new bubble formation are the evidence for a chemical reaction occuring. Not every time one of these changes is proof of a chemical reaction, but often they are. Sometimes chemical reactions can occur with no o ...
3.1.2 Group 2
... energy to remove these electrons are the first and second ionisation energy The first ionisation energy is Energy needed to remove an electron from each atom in one mole of gaseous atoms This is represented by the equation: ...
... energy to remove these electrons are the first and second ionisation energy The first ionisation energy is Energy needed to remove an electron from each atom in one mole of gaseous atoms This is represented by the equation: ...
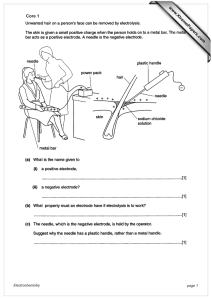
Core 1 www.XtremePapers.com Electrochemistry page 1
... produces electrical energy or voltage or current from chemical energy or chemical reactions or two different electrodes in electrolyte ...
... produces electrical energy or voltage or current from chemical energy or chemical reactions or two different electrodes in electrolyte ...
Atomic Theories and Models - MrD-Home
... version of the _________ element (isotope) will be produced. • e.g. ...
... version of the _________ element (isotope) will be produced. • e.g. ...
Chem 1100 Chapter Three Study Guide Outline I. Molar Mass and
... 20. Sodium metal and water react to form hydrogen and sodium hydroxide. If 5.98 g of sodium react with water to form 0.26 g of hydrogen and 10.40 g of sodium hydroxide, what mass of water was consumed in the reaction? a. 10.66 g b. 4.68 g c. 10.14 g d. 5.98 g 21. What is the chemical formula for str ...
... 20. Sodium metal and water react to form hydrogen and sodium hydroxide. If 5.98 g of sodium react with water to form 0.26 g of hydrogen and 10.40 g of sodium hydroxide, what mass of water was consumed in the reaction? a. 10.66 g b. 4.68 g c. 10.14 g d. 5.98 g 21. What is the chemical formula for str ...
File
... • Each group should have 4 people and 20 cards. Split the cards up so each person has 5. • On your own piece of paper, write out your 5 chemical equations and balance them. • Then, once all equations are balanced, look at the 20 as a group. You need to split the 20 cards up in to 5 different react ...
... • Each group should have 4 people and 20 cards. Split the cards up so each person has 5. • On your own piece of paper, write out your 5 chemical equations and balance them. • Then, once all equations are balanced, look at the 20 as a group. You need to split the 20 cards up in to 5 different react ...
H H H H H N HO O NC[ ]- - Teacher`s Tools® Chemistry
... Transition metals can behave like Lewis Acids (electron pair acceptors) in the formation of what are called complex ions. A coordination compound typically consists of a complex ion and counter ion. The molecules or ions that surround the metal in a complex ion are called ligands. The ligands act li ...
... Transition metals can behave like Lewis Acids (electron pair acceptors) in the formation of what are called complex ions. A coordination compound typically consists of a complex ion and counter ion. The molecules or ions that surround the metal in a complex ion are called ligands. The ligands act li ...
mod 2 revision guide 6. group 2
... An equation for the formation of the precipitate can be written as a full equation or simplest ionic equation Full equation : SrCl2(aq) + Na2SO4 (aq) 2NaCl (aq) + SrSO4 (s) Ionic equation: Sr2+ (aq) + SO42-(aq) SrSO4 (s). BaSO4 is used in medicine as a ‘Barium meal’ given to patients who need x-rays ...
... An equation for the formation of the precipitate can be written as a full equation or simplest ionic equation Full equation : SrCl2(aq) + Na2SO4 (aq) 2NaCl (aq) + SrSO4 (s) Ionic equation: Sr2+ (aq) + SO42-(aq) SrSO4 (s). BaSO4 is used in medicine as a ‘Barium meal’ given to patients who need x-rays ...
CHEM_1305_Practice_Exam_2
... 17) What is the name of a reaction in which two cations in different compounds exchange anions? A) combination ...
... 17) What is the name of a reaction in which two cations in different compounds exchange anions? A) combination ...
UNIT 1 - MATTER AND CHEMICAL BONDING
... 2. Be able to draw and interpret a graph pertaining to Boyle’s Law. 3. Describe the quantitative relationships which exist among the following variables for an ideal gas (P, V, T, n) (e.g. P T with V & n constant) 4. What is an ideal gas? Under what conditions does a real gas behave most like an i ...
... 2. Be able to draw and interpret a graph pertaining to Boyle’s Law. 3. Describe the quantitative relationships which exist among the following variables for an ideal gas (P, V, T, n) (e.g. P T with V & n constant) 4. What is an ideal gas? Under what conditions does a real gas behave most like an i ...
examreview_June2010 - St. Mary CSS Chemistry11U 2010
... 2. a) Be able to describe the motion of particles in the gaseous state using the kinetic molecular theory. b) Use this type of description to describe the changes in volume, or pressure of a gas if another variable (V, P or T) is changed. 3. Describe the quantitative relationships which exist among ...
... 2. a) Be able to describe the motion of particles in the gaseous state using the kinetic molecular theory. b) Use this type of description to describe the changes in volume, or pressure of a gas if another variable (V, P or T) is changed. 3. Describe the quantitative relationships which exist among ...
Intro to Chemistry
... The dark bottle in which hydrogen peroxide is usually stored keeps out the light, thus protecting the H2O2 from decomposition. ...
... The dark bottle in which hydrogen peroxide is usually stored keeps out the light, thus protecting the H2O2 from decomposition. ...
Exam Review
... pairs of acids and bases. a) sulphuric acid + sodium hydroxide HsSO4(aq) NaOH(aq) b) hydrochloric acid + calcium hydroxide HCl(aq) Ca(OH)2(aq) c) phosphoric acid + sodium hydroxide H3PO4(aq) NaOH(aq) d) nitric acid + potassium hydroxide HNO3(aq) + KOH(aq) ...
... pairs of acids and bases. a) sulphuric acid + sodium hydroxide HsSO4(aq) NaOH(aq) b) hydrochloric acid + calcium hydroxide HCl(aq) Ca(OH)2(aq) c) phosphoric acid + sodium hydroxide H3PO4(aq) NaOH(aq) d) nitric acid + potassium hydroxide HNO3(aq) + KOH(aq) ...
Take Home - mvhs
... (2 pts) Write a balanced equation for the reaction between UF6 and H2O. Assume that the empirical formula of the gas is the true formula. ...
... (2 pts) Write a balanced equation for the reaction between UF6 and H2O. Assume that the empirical formula of the gas is the true formula. ...
Grade 11 Chemistry E.. - hrsbstaff.ednet.ns.ca
... g. Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + NaCl(aq) h. CH3OH(l) + O2(g) → CO2(g) + H2O(g) 25. Classify each of the above according to the 5 types of reactions (composition, decomposition, single replacement, double replacement and combustion). 26. Write the formula for each material correctly and then b ...
... g. Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + NaCl(aq) h. CH3OH(l) + O2(g) → CO2(g) + H2O(g) 25. Classify each of the above according to the 5 types of reactions (composition, decomposition, single replacement, double replacement and combustion). 26. Write the formula for each material correctly and then b ...
2016 Pre Course CHEMISTRY - Calday Grange Grammar School
... molten. Carbon disulphide, CS2, is a liquid which does not conduct electricity. (a) ...
... molten. Carbon disulphide, CS2, is a liquid which does not conduct electricity. (a) ...
Mr. B`s Chemistry
... Write formulas for the reactants and predicted products for the chemical reactions that follow. Assume that in all cases a reaction occurs. The equation must be balanced. Write all substances in their proper form –as ions if appropriate – and cancel any spectator ions. ...
... Write formulas for the reactants and predicted products for the chemical reactions that follow. Assume that in all cases a reaction occurs. The equation must be balanced. Write all substances in their proper form –as ions if appropriate – and cancel any spectator ions. ...
CHAPTER 6: Earth science
... Predicting precipitation 2. The following table provides information on the solubility of various salts in water. Anion ...
... Predicting precipitation 2. The following table provides information on the solubility of various salts in water. Anion ...
Sodium hydroxide

Sodium hydroxide (NaOH), also known as lye and caustic soda, is an inorganic compound. It is a white solid and highly caustic metallic base and alkali salt which is available in pellets, flakes, granules, and as prepared solutions at a number of different concentrations. Sodium hydroxide forms an approximately 50% (by weight) saturated solution with water.Sodium hydroxide is soluble in water, ethanol and methanol. This alkali is deliquescent and readily absorbs moisture and carbon dioxide in air.Sodium hydroxide is used in many industries, mostly as a strong chemical base in the manufacture of pulp and paper, textiles, drinking water, soaps and detergents and as a drain cleaner. Worldwide production in 2004 was approximately 60 million tonnes, while demand was 51 million tonnes.











![H H H H H N HO O NC[ ]- - Teacher`s Tools® Chemistry](http://s1.studyres.com/store/data/017018154_1-68467b392d8cadb27de319df72045839-300x300.png)