Chapter 5 Review Game Questions
... 14) Where are the p block elements on the periodic table? (right) 15) Where is the d-block on the periodic table? (middle) 16) Where is the s block on the periodic table? (left) 17) Half the distance between the nuclei of identical atoms that are bonded together? (atomic radius) 18) Elements on the ...
... 14) Where are the p block elements on the periodic table? (right) 15) Where is the d-block on the periodic table? (middle) 16) Where is the s block on the periodic table? (left) 17) Half the distance between the nuclei of identical atoms that are bonded together? (atomic radius) 18) Elements on the ...
Chapter 5 Review Sheet Be sure to study the following vocabulary
... Be sure to study the following vocabulary: Valence electron- the electrons in the outermost energy level of an atom Periodic Table- an arrangement of the elements according to their atomic numbers Alkali Metals- the elements in Group 1 of the periodic table; they are the most reactive; their atoms h ...
... Be sure to study the following vocabulary: Valence electron- the electrons in the outermost energy level of an atom Periodic Table- an arrangement of the elements according to their atomic numbers Alkali Metals- the elements in Group 1 of the periodic table; they are the most reactive; their atoms h ...
Ex. 06 Answer
... a) The reactivity of Group 1 elements increases down the group. b) To prevent it from reacting with the air. c) i) Their atoms have the same number of outermost shell electrons. ii) Their atoms have different number of occupied electron shells. d) • Potassium floats on the surface of water while mag ...
... a) The reactivity of Group 1 elements increases down the group. b) To prevent it from reacting with the air. c) i) Their atoms have the same number of outermost shell electrons. ii) Their atoms have different number of occupied electron shells. d) • Potassium floats on the surface of water while mag ...
Prentice Hall Physical Science Chapter 5: The Periodic Table
... *** Valence electrons are the number of electrons in the outermost energy level of the atom. *** Elements in the same group have similar properties because they have the same number of valence electrons. Group 1 (1A)- Alkali Metals (p. 140) 1 valence electron soft, silver-white, shiny metals good co ...
... *** Valence electrons are the number of electrons in the outermost energy level of the atom. *** Elements in the same group have similar properties because they have the same number of valence electrons. Group 1 (1A)- Alkali Metals (p. 140) 1 valence electron soft, silver-white, shiny metals good co ...
WILF 1 - GCSE Chemistry Help
... If elements are arranged in increasing order of atomic number, the problems with elements like tellurium and iodine are solved. Elements in the same group have the same number of outer shell electrons. It is the number of outer shell electrons which determines how the element behaves. Chlorine ...
... If elements are arranged in increasing order of atomic number, the problems with elements like tellurium and iodine are solved. Elements in the same group have the same number of outer shell electrons. It is the number of outer shell electrons which determines how the element behaves. Chlorine ...
The Periodic Table
... have similar but not In fact, the properties identical properties. change greatly across even For example, lithium (Li), given row. sodium (Na), potassium (K), and other members of The first element in a period is always an group IA are all soft, white, extremely active solid. The shiny metals ...
... have similar but not In fact, the properties identical properties. change greatly across even For example, lithium (Li), given row. sodium (Na), potassium (K), and other members of The first element in a period is always an group IA are all soft, white, extremely active solid. The shiny metals ...
The Periodic Table
... have similar but not In fact, the properties identical properties. change greatly across even For example, lithium (Li), given row. sodium (Na), potassium (K), and other members of The first element in a period is always an group IA are all soft, white, extremely active solid. The shiny metals ...
... have similar but not In fact, the properties identical properties. change greatly across even For example, lithium (Li), given row. sodium (Na), potassium (K), and other members of The first element in a period is always an group IA are all soft, white, extremely active solid. The shiny metals ...
Element Review
... Elements in the same family have similar… Properties or reactions What is the only element that does not fit into any family? Hydrogen ...
... Elements in the same family have similar… Properties or reactions What is the only element that does not fit into any family? Hydrogen ...
File
... 2. Identify each element as a metal, metalloid, or nonmetal. a) fluorine b) germanium c) zinc d) phosphorous e) lithium 3. Give two examples of elements for each category. a) noble gases b) halogens c) alkali metals d) alkaline earth metals 4. The halogen family or halides form salts with which othe ...
... 2. Identify each element as a metal, metalloid, or nonmetal. a) fluorine b) germanium c) zinc d) phosphorous e) lithium 3. Give two examples of elements for each category. a) noble gases b) halogens c) alkali metals d) alkaline earth metals 4. The halogen family or halides form salts with which othe ...
Ch.4 Notes Powerpoint Version
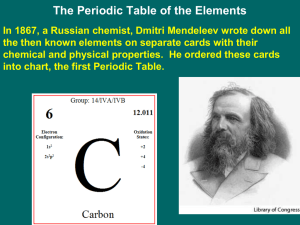
... • Dmitri Mendeleev found that by placing elements in order of increasing atomic mass properties of elements were repeated. ▫ Each new row = properties repeated. ▫ This resulted in each column having elements with similar properties. ...
... • Dmitri Mendeleev found that by placing elements in order of increasing atomic mass properties of elements were repeated. ▫ Each new row = properties repeated. ▫ This resulted in each column having elements with similar properties. ...
Chapter 12: Chemical Periodicity
... elements discovered were copper, silver and gold. This occurred approximately eight thousand years ago. Lead, tin, iron, mercury, carbon and sulfur were discovered next. As people began working with metals, the Alchemists searched for a method of turning lead (or other ordinary metals) into gold. Th ...
... elements discovered were copper, silver and gold. This occurred approximately eight thousand years ago. Lead, tin, iron, mercury, carbon and sulfur were discovered next. As people began working with metals, the Alchemists searched for a method of turning lead (or other ordinary metals) into gold. Th ...
The Periodic Table of Elements
... They are highly reactive but are slightly less reactive and harder than the Group 1 metals. The reactivity of both Group 1 and 2 metals increases with increasing atomic number. ...
... They are highly reactive but are slightly less reactive and harder than the Group 1 metals. The reactivity of both Group 1 and 2 metals increases with increasing atomic number. ...
2.2 Periodic Chart
... distinctive colours (Ne is reddish) when electricity is passed through them. Their ion charges of zero indicate that they do not form charged ions. ...
... distinctive colours (Ne is reddish) when electricity is passed through them. Their ion charges of zero indicate that they do not form charged ions. ...
Section 15.1
... Remember, the atomic number is the number of protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
... Remember, the atomic number is the number of protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
UNIT 6: PERIODICITY THE FOREST: Repeating (periodic) patterns
... Describe the locations in the periodic table and the general properties of the alkali metals, the alkaline earth metals, the halogens, and the noble gases. Use the periodic table to predict the electron configurations of elements. Give examples of the relationship between an element’s electron confi ...
... Describe the locations in the periodic table and the general properties of the alkali metals, the alkaline earth metals, the halogens, and the noble gases. Use the periodic table to predict the electron configurations of elements. Give examples of the relationship between an element’s electron confi ...
The Periodic Table and Trends of the Elements
... with an oxidation state of +1. This is similar to the alkali metal group so it is placed at the top of that group. Sometime, however it is placed by itself at the middle of the periodic table to indicate its uniqueness. ...
... with an oxidation state of +1. This is similar to the alkali metal group so it is placed at the top of that group. Sometime, however it is placed by itself at the middle of the periodic table to indicate its uniqueness. ...
The Periodic Table - Ms. Dormer
... gaps that unknown elements should fill He predicted the properties of these unknown elements & gave them names ...
... gaps that unknown elements should fill He predicted the properties of these unknown elements & gave them names ...
Chapter 6 Study Guide
... b. The inner transition metal with the lowest atomic number c. A metal in group 5A d. All the transition metals with an atomic number that is a multiple of 5 e. The electron configuration ends with 4s2 ...
... b. The inner transition metal with the lowest atomic number c. A metal in group 5A d. All the transition metals with an atomic number that is a multiple of 5 e. The electron configuration ends with 4s2 ...
noble gases
... proton and no neutron in its nucleus. Hydrogen doesn’t have much in common with the alkali metals. It’s a colourless, odourless, tasteless, highly flammable gas. Almost all of Earth’s hydrogen exists in combination with other elements. Its reactivity is too great for it to exist in the atmosphere as ...
... proton and no neutron in its nucleus. Hydrogen doesn’t have much in common with the alkali metals. It’s a colourless, odourless, tasteless, highly flammable gas. Almost all of Earth’s hydrogen exists in combination with other elements. Its reactivity is too great for it to exist in the atmosphere as ...
periodic table power point
... with an oxidation state of +1. This is similar to the alkali metal group so it is placed at the top of that group. Sometime, however it is placed by itself at the middle of the periodic table to indicate its uniqueness. ...
... with an oxidation state of +1. This is similar to the alkali metal group so it is placed at the top of that group. Sometime, however it is placed by itself at the middle of the periodic table to indicate its uniqueness. ...
Unit 2 Periodic Table
... make up our bodies, our world, our sun, and in fact, the entire universe. ...
... make up our bodies, our world, our sun, and in fact, the entire universe. ...
Next > Mendeleev and Meyer
... extremely un-reactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. All the noble gases are found in small am ...
... extremely un-reactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. All the noble gases are found in small am ...
Periodic trends Tempura
... Mendeleev said that the properties of the elements are periodic if elements are arranged by increasing atomic mass. The use of mass was incorrect as Mendeleev found with the discovery of reversed pairs. Modern periodic law says the properties are periodic (and elements are in the same column if they ...
... Mendeleev said that the properties of the elements are periodic if elements are arranged by increasing atomic mass. The use of mass was incorrect as Mendeleev found with the discovery of reversed pairs. Modern periodic law says the properties are periodic (and elements are in the same column if they ...