Formation of C-C Bonds via Catalytic Hydrogenation and Transfer
... laboratory reveals reductive C-C bond formation can be achieved under the conditions of catalytic hydrogenation. This concept is extended further via “C-C bond forming transfer hydrogenations”, wherein hydrogen exchange between alcohols and π-unsaturated reactants triggers generation of aldehyde-org ...
... laboratory reveals reductive C-C bond formation can be achieved under the conditions of catalytic hydrogenation. This concept is extended further via “C-C bond forming transfer hydrogenations”, wherein hydrogen exchange between alcohols and π-unsaturated reactants triggers generation of aldehyde-org ...
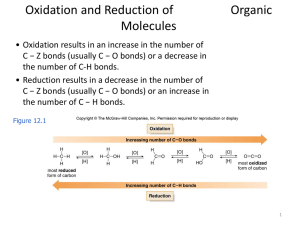
Oxidation and Reduction of Organic Molecules
... hydrogenation to distinguish the nature of unsaturation in a molecule. • This is done by comparing the degrees of unsaturation before and after a molecule is treated with H2. • The number of degrees of unsaturation lost = the number of bonds. • The number of degrees of unsaturation remaining in th ...
... hydrogenation to distinguish the nature of unsaturation in a molecule. • This is done by comparing the degrees of unsaturation before and after a molecule is treated with H2. • The number of degrees of unsaturation lost = the number of bonds. • The number of degrees of unsaturation remaining in th ...
Hydrogenation

Hydrogenation – to treat with hydrogen – is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, generally an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.Because of the importance of hydrogen, many related reactions have been developed for its use. Most hydrogenations use gaseous hydrogen (H2), but some involve the alternative sources of hydrogen, not H2: these processes are called transfer hydrogenations. The reverse reaction, removal of hydrogen from a molecule, is called dehydrogenation. A reaction where bonds are broken while hydrogen is added is called hydrogenolysis, a reaction that may occur to carbon-carbon and carbon-heteroatom (oxygen, nitrogen or halogen) bonds. Hydrogenation differs from protonation or hydride addition: in hydrogenation, the products have the same charge as the reactants.Hydrogenation of unsaturated fats produces saturated fats. In the case of partial hydrogenation, trans fats may be generated as well.