Flash Calculations - Rowan University
... Note: For the process simulator this problem is over specified! In a process simulator the equations that are used include n mass balances, 1 energy balance and n+2 phase equilibrium equations, where n is the number of chemical species. A typical set of specifications for this problem is to fully sp ...
... Note: For the process simulator this problem is over specified! In a process simulator the equations that are used include n mass balances, 1 energy balance and n+2 phase equilibrium equations, where n is the number of chemical species. A typical set of specifications for this problem is to fully sp ...
chapter 1 - Louisiana Tech University
... matter (solid, liquid, gas) observed for a particular substance. (2) The components of a mixture maintain their identity if the mixture is heterogeneous but lose their identity if the mixture is homogeneous. (3) No more than six atoms may be present in a heteroatomic molecule. a) All three statement ...
... matter (solid, liquid, gas) observed for a particular substance. (2) The components of a mixture maintain their identity if the mixture is heterogeneous but lose their identity if the mixture is homogeneous. (3) No more than six atoms may be present in a heteroatomic molecule. a) All three statement ...
STOICHIOMETRY:
... 4. Using the molar mass of the unknown substance, convert the moles just calculated to mass. Mass of hydrogen: ...
... 4. Using the molar mass of the unknown substance, convert the moles just calculated to mass. Mass of hydrogen: ...
Paper
... The curves obtained by the approximation using equations (5), (11) and (12) are shown in Fig.4 together with the reference experimental points. The system (5) includes 4 different energy parameters. Since at high values of these parameters, typical for oxide melts, the excess chemical potentials exp ...
... The curves obtained by the approximation using equations (5), (11) and (12) are shown in Fig.4 together with the reference experimental points. The system (5) includes 4 different energy parameters. Since at high values of these parameters, typical for oxide melts, the excess chemical potentials exp ...
The Mayer-Joule Principle: The Foundation of
... and Carnot could produce work from heat (albeit not with 100% efficiency), no one had appreciated that heat and work were different aspects of the same physical entity. What then did Mayer propose? As a physician in Java, he observed that venous blood drawn in the tropics was far brighter than that ...
... and Carnot could produce work from heat (albeit not with 100% efficiency), no one had appreciated that heat and work were different aspects of the same physical entity. What then did Mayer propose? As a physician in Java, he observed that venous blood drawn in the tropics was far brighter than that ...
BOLTZMANN ENTROPY: PROBABILITY AND INFORMATION The
... The concept of entropy was first introduced in thermodynamics by Clausius through the second law of thermodynamics. It was Boltzmann who first provided the statistical analogue of thermodynamic entropy linking the concept of entropy with molecular disorder or chaos [1]. The concept of entropy was la ...
... The concept of entropy was first introduced in thermodynamics by Clausius through the second law of thermodynamics. It was Boltzmann who first provided the statistical analogue of thermodynamic entropy linking the concept of entropy with molecular disorder or chaos [1]. The concept of entropy was la ...
9 free IB Chem labs (sent to OCC) - VicPark-IBRoundtable-2009
... 8. Repeat this experiment a total of 3 times. Data Collection and Processing: Since we are using data logging software, you must be careful to take the raw data and present it in your own words and your own table. ...
... 8. Repeat this experiment a total of 3 times. Data Collection and Processing: Since we are using data logging software, you must be careful to take the raw data and present it in your own words and your own table. ...
Lecture 4
... TIP Whenever the amounts of two or more reactants are given, you must do calculations for each reactant to determine whether each is completely consumed!!! (You can also calculate afterwards how much of the other reactants are left-over.) There are (at least) two ways that you can use to calculate w ...
... TIP Whenever the amounts of two or more reactants are given, you must do calculations for each reactant to determine whether each is completely consumed!!! (You can also calculate afterwards how much of the other reactants are left-over.) There are (at least) two ways that you can use to calculate w ...
- Kendriya Vidyalaya No. 2 Raipur
... Mohan took pure water for the electrolytic decomposition of water but did not see any bubbles near the electrodes. Explain why? Rancidity is a process used for spoiling of cooked food materials like vegetables, etc. When kept for long time in open. How can you prevent such process to proceed? Give a ...
... Mohan took pure water for the electrolytic decomposition of water but did not see any bubbles near the electrodes. Explain why? Rancidity is a process used for spoiling of cooked food materials like vegetables, etc. When kept for long time in open. How can you prevent such process to proceed? Give a ...
File
... Mohan took pure water for the electrolytic decomposition of water but did not see any bubbles near the electrodes. Explain why? Rancidity is a process used for spoiling of cooked food materials like vegetables, etc. When kept for long time in open. How can you prevent such process to proceed? Give a ...
... Mohan took pure water for the electrolytic decomposition of water but did not see any bubbles near the electrodes. Explain why? Rancidity is a process used for spoiling of cooked food materials like vegetables, etc. When kept for long time in open. How can you prevent such process to proceed? Give a ...
14.1 The Atmosphere and Atmospheric chemistry
... significant levels of electrons and positive ions. Because of the rarefied conditions, these ions may exist in the upper atmosphere for long periods before recombining to form neutral species. At altitudes of approximately 50 km and up, ions are so prevalent that the region is called the ionosphere. ...
... significant levels of electrons and positive ions. Because of the rarefied conditions, these ions may exist in the upper atmosphere for long periods before recombining to form neutral species. At altitudes of approximately 50 km and up, ions are so prevalent that the region is called the ionosphere. ...
Carefully detach the last page. It is the Data Sheet.
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
CHAPTER 6
... Notably, this equation can be also derived starting from an overall mass balance similar to that expressed by eq.(4.46) assuming, Gi = 0, J X Ci vX , J Y Ci vY and J Z Ci vZ . To conclude this paragraph, it is important to remember that mass diffusion is not only due to the presence of the con ...
... Notably, this equation can be also derived starting from an overall mass balance similar to that expressed by eq.(4.46) assuming, Gi = 0, J X Ci vX , J Y Ci vY and J Z Ci vZ . To conclude this paragraph, it is important to remember that mass diffusion is not only due to the presence of the con ...
pdf version - Joliet Junior College
... Summary: Recall that ‘Nature likes stable’! Or, in other words, the ‘winner’ (in terms of the relative strengths of respective intermolecular force combinations) typically determines whether the solute /solvent pair are soluble or insoluble. ...
... Summary: Recall that ‘Nature likes stable’! Or, in other words, the ‘winner’ (in terms of the relative strengths of respective intermolecular force combinations) typically determines whether the solute /solvent pair are soluble or insoluble. ...
MS PowerPoint - Catalysis Eprints database
... dipole. It has the following properties: Non polar molecules acquire and induced dipole moment in an electric field and this induced moment is only temporary and disappears as soon as the field is removed. The field maybe due to the presence of nearby dipoles. The average interaction energy comes ou ...
... dipole. It has the following properties: Non polar molecules acquire and induced dipole moment in an electric field and this induced moment is only temporary and disappears as soon as the field is removed. The field maybe due to the presence of nearby dipoles. The average interaction energy comes ou ...
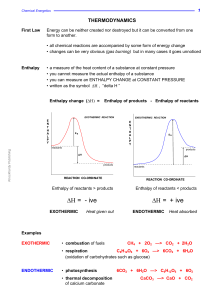
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.