Chapter 7: Recent advances in enzyme technology
... of hydrolysis will not be affected as the reaction proceeds. By greatly reducing the water activity in these systems they can be used to transfer to other acceptors. Examples of this can be found in the transesterification reactions of esterases and lipases, described more fully later, and the (unde ...
... of hydrolysis will not be affected as the reaction proceeds. By greatly reducing the water activity in these systems they can be used to transfer to other acceptors. Examples of this can be found in the transesterification reactions of esterases and lipases, described more fully later, and the (unde ...
AP Chemistry Syllabus - Old Mill High School
... on the AP Chemistry exam and to be equivalent to the general chemistry course usually taken during the first year of college. Topics include structure of matter, kinetic theory of gases, chemical equilibria, chemical kinetics, and thermodynamics. An emphasis is placed on chemical calculations, mathe ...
... on the AP Chemistry exam and to be equivalent to the general chemistry course usually taken during the first year of college. Topics include structure of matter, kinetic theory of gases, chemical equilibria, chemical kinetics, and thermodynamics. An emphasis is placed on chemical calculations, mathe ...
Chemistry 12 Worksheet 2-3 Calculations Involving the
... PDFMAILER.COM Print and send PDF files as Emails with any application, ad-sponsored and free of charge www.pdfmailer.com ...
... PDFMAILER.COM Print and send PDF files as Emails with any application, ad-sponsored and free of charge www.pdfmailer.com ...
Chemistry and electronic properties of metal
... briefly mentioned here and will be reported in detail elsewhere.14 Because PTCDA is known as a hole-transport medium, the alignment of E F with filled molecular levels at the OMTF surface is a priori thought to be a determining factor in the electronic properties of the interface. This issue is inve ...
... briefly mentioned here and will be reported in detail elsewhere.14 Because PTCDA is known as a hole-transport medium, the alignment of E F with filled molecular levels at the OMTF surface is a priori thought to be a determining factor in the electronic properties of the interface. This issue is inve ...
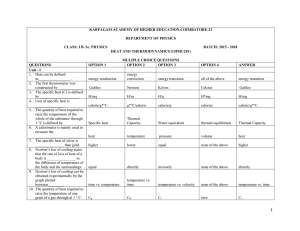
Heat and Thermodynamics 300 MCQ
... unit volume E is equal to 57. The unit of power in SI units is 58. The ratio of two specific heats of air is equal to 59. Which law states that the internal energy of a gas is a function of temperature 60. Which law states that the specific heat of a gas remains constant at all temperature and press ...
... unit volume E is equal to 57. The unit of power in SI units is 58. The ratio of two specific heats of air is equal to 59. Which law states that the internal energy of a gas is a function of temperature 60. Which law states that the specific heat of a gas remains constant at all temperature and press ...
2 - mrstorie
... a. What is the equilibrium constant expression for this reaction? Kc = [NO]2/[NO]2[O2] b. What is the equilibrium constant for the reaction? 6.4 x 105 7. Phosphorous pentachloride decomposes to phosphorous trichloride according to this equation: PCl5 (g) PCl3 (g) + Cl2 (g) Keq = 1.00 x 10-3 At eq ...
... a. What is the equilibrium constant expression for this reaction? Kc = [NO]2/[NO]2[O2] b. What is the equilibrium constant for the reaction? 6.4 x 105 7. Phosphorous pentachloride decomposes to phosphorous trichloride according to this equation: PCl5 (g) PCl3 (g) + Cl2 (g) Keq = 1.00 x 10-3 At eq ...
1. Explain electrophile and nucleophile. 2. Explain
... 41. Explain the construction and working of Bomb Calorimeter. 42. A system absorbs 52kj heat from a heat reservoir and 74 KJ work is done on it by the surroundings. What is the change in internal energy of the system? 43. Explain the relationship between DH and DU. 44. Calculate ...
... 41. Explain the construction and working of Bomb Calorimeter. 42. A system absorbs 52kj heat from a heat reservoir and 74 KJ work is done on it by the surroundings. What is the change in internal energy of the system? 43. Explain the relationship between DH and DU. 44. Calculate ...
Colligative Properties
... The treatment of osmotic pressure is not identical to the treatments of boiling point elevation and freezing point depression we have just given. A cartoon of an osmotic pressure apparatus is shown below. A U-tube has a "semipermeable membrane inserted in the center of the cross piece as shown. (A s ...
... The treatment of osmotic pressure is not identical to the treatments of boiling point elevation and freezing point depression we have just given. A cartoon of an osmotic pressure apparatus is shown below. A U-tube has a "semipermeable membrane inserted in the center of the cross piece as shown. (A s ...
Chemistry Higher Level Chapter 5 - Pearson Schools and FE Colleges
... of knowledge? Is their correct use a necessary or sufficient indicator of understanding? ...
... of knowledge? Is their correct use a necessary or sufficient indicator of understanding? ...
5 Energetics - Pearson Schools and FE Colleges
... of knowledge? Is their correct use a necessary or sufficient indicator of understanding? ...
... of knowledge? Is their correct use a necessary or sufficient indicator of understanding? ...
chemistry
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
chapter 16
... You must check validity by plugging "x" over original concentration. It must be less than 5% of the original concentration to be valid. 2. If "x" is necessary, then see if the problem may be a perfect square and thus, ease the steps of solving. (Sometimes you must use the quadratic formula!) 3. If n ...
... You must check validity by plugging "x" over original concentration. It must be less than 5% of the original concentration to be valid. 2. If "x" is necessary, then see if the problem may be a perfect square and thus, ease the steps of solving. (Sometimes you must use the quadratic formula!) 3. If n ...
Molecules, Moles and Chemical Equations File
... and safer to handle (Figure 3.1). In the chemical reactions associated with explosions, the solid explosive is converted predominantly into gases. This is no coincidence; the generation of gases is actually very important to the development of the explosion. When a chemical reaction transforms a sol ...
... and safer to handle (Figure 3.1). In the chemical reactions associated with explosions, the solid explosive is converted predominantly into gases. This is no coincidence; the generation of gases is actually very important to the development of the explosion. When a chemical reaction transforms a sol ...
Topic 5 Energetics File
... Enthalpy: The internal energy stored in the reactants. Only changes in enthalpy can be measured. Entropy: A measure of the disorder of a system. Things causing entropy to increase: 1) increase of number of moles of gaseous molecules; 2) change of state from solid to liquid or liquid to gas; 3) incre ...
... Enthalpy: The internal energy stored in the reactants. Only changes in enthalpy can be measured. Entropy: A measure of the disorder of a system. Things causing entropy to increase: 1) increase of number of moles of gaseous molecules; 2) change of state from solid to liquid or liquid to gas; 3) incre ...
Chapter 3
... (as Ca2+ ions). How many moles of calcium are in each tablet? (The average atomic mass of an atom of calcium is 40.078 amu, which means the molar mass of calcium is 40.08 g/mol when rounded to four significant figures.1 ) ...
... (as Ca2+ ions). How many moles of calcium are in each tablet? (The average atomic mass of an atom of calcium is 40.078 amu, which means the molar mass of calcium is 40.08 g/mol when rounded to four significant figures.1 ) ...
Chapter Six
... The internal energy of a fixed quantity of an ideal gas depends only on its temperature. If a sample of an ideal gas is allowed to expand against a constant pressure at a constant temperature, (a) what is DU for the gas? (b) Does the gas do work? (c) Is any heat exchanged with the surroundings? ...
... The internal energy of a fixed quantity of an ideal gas depends only on its temperature. If a sample of an ideal gas is allowed to expand against a constant pressure at a constant temperature, (a) what is DU for the gas? (b) Does the gas do work? (c) Is any heat exchanged with the surroundings? ...
Here`s - Sonlight
... What is the chemical name for the ionic compound CuCl2? Step 1: Identify the cation and anion and their charges. To solve this problem, we first need to determine the charges that Cu and Cl develop in an ionic compound. Cl is in group 7A, so it wants to be 1-. Cu is in group 8B, so it can possibly h ...
... What is the chemical name for the ionic compound CuCl2? Step 1: Identify the cation and anion and their charges. To solve this problem, we first need to determine the charges that Cu and Cl develop in an ionic compound. Cl is in group 7A, so it wants to be 1-. Cu is in group 8B, so it can possibly h ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.