Chapter 3 – Stoichiometry of Formulas and Equations This chapter
... while C & D are products. Sometimes the symbols (heat) or h (light) are placed above the arrow to indicate if outside sources of energy were used to facilitate the reaction. Also, sometimes the solvent (section 4.1) is placed below the arrow. Usually the physical state of each species will be ind ...
... while C & D are products. Sometimes the symbols (heat) or h (light) are placed above the arrow to indicate if outside sources of energy were used to facilitate the reaction. Also, sometimes the solvent (section 4.1) is placed below the arrow. Usually the physical state of each species will be ind ...
The Process of Chemical Reactions
... absorb energy overall as it takes place. If this energy comes from the motion (kinetic energy) of the reactants, the particles in the system will be moving more slowly after the reaction than before. The system will have lower kinetic energy, and the temperature will decrease. Because the system is ...
... absorb energy overall as it takes place. If this energy comes from the motion (kinetic energy) of the reactants, the particles in the system will be moving more slowly after the reaction than before. The system will have lower kinetic energy, and the temperature will decrease. Because the system is ...
2005 - NESACS
... 63. From the thermodynamic values given below, the lattice energy for BaCl2 is (A) 1010 kJ/mole (B) 2050 kJ/mole (C) 1530 kJ/mole (D) 1360 kJ/mole ...
... 63. From the thermodynamic values given below, the lattice energy for BaCl2 is (A) 1010 kJ/mole (B) 2050 kJ/mole (C) 1530 kJ/mole (D) 1360 kJ/mole ...
rate law determination of crystal violet hydroxylation
... Do not round off value for n if it is a decimal or fraction. Report this value to 2 sig figs. (The concentration of OH– was 0.020 M in Part A and 0.010 M in Part B.) (8) Now round off the value for n to an integer (0, 1, 2, etc.). Write the complete rate law expression using both CV+ and OH– in the ...
... Do not round off value for n if it is a decimal or fraction. Report this value to 2 sig figs. (The concentration of OH– was 0.020 M in Part A and 0.010 M in Part B.) (8) Now round off the value for n to an integer (0, 1, 2, etc.). Write the complete rate law expression using both CV+ and OH– in the ...
Unit 5 Student Packet
... (a) Calculate the ∆H˚ for the reaction above, using the table of average bond dissociation energies. (b) Calculate the ∆S˚ for the reaction at 298 K, using data from either table as needed. (c) Calculate the value of Keq for the reaction at 298 K. (d) What is the effect of an increase in temperature ...
... (a) Calculate the ∆H˚ for the reaction above, using the table of average bond dissociation energies. (b) Calculate the ∆S˚ for the reaction at 298 K, using data from either table as needed. (c) Calculate the value of Keq for the reaction at 298 K. (d) What is the effect of an increase in temperature ...
A) 0% B) 20% C) 50% D) 80% E) 100% 1. Naturally occurring boron
... Low pressure and low temperature Low pressure and high temperature High pressure and high temperature High pressure and low density Low temperature and high density ...
... Low pressure and low temperature Low pressure and high temperature High pressure and high temperature High pressure and low density Low temperature and high density ...
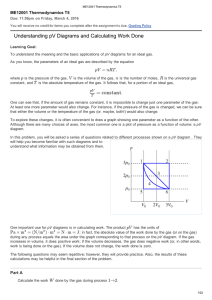
Understanding pV Diagrams and Calculating Work Done
... The process illustrated here is somewhat different from those we have been studying, because the pressure change is due to changes in the amount of gas in the lung, not to temperature changes. (Think of your own breathing. Your lungs do not expand because they've gotten hot.) If the temperature of t ...
... The process illustrated here is somewhat different from those we have been studying, because the pressure change is due to changes in the amount of gas in the lung, not to temperature changes. (Think of your own breathing. Your lungs do not expand because they've gotten hot.) If the temperature of t ...
Surface chemistry and Catalysis
... Adsorption is accompanied by decrease in the ΔG (free energy) of the system when ΔG = 0 adsorption equilibrium is said to be established. Adsorption is invariably accompanied by evolution of heat ie. It is an exothermic process. In other words ΔH of adsorption is always negative. When a gas is ...
... Adsorption is accompanied by decrease in the ΔG (free energy) of the system when ΔG = 0 adsorption equilibrium is said to be established. Adsorption is invariably accompanied by evolution of heat ie. It is an exothermic process. In other words ΔH of adsorption is always negative. When a gas is ...
Document
... 4. Given the chemical equation 2NI3(s) N2(g) + 3I2(g), which of the following descriptions of the reaction are correct? Select all correct answers. a. 1 mole of NI3 decomposes to produce 0.5 moles of N2 and 1.5 moles of I2 b. 2 moles of NI3 decomposes to produce 1 mole of N2 and 3 moles of I2. c. ...
... 4. Given the chemical equation 2NI3(s) N2(g) + 3I2(g), which of the following descriptions of the reaction are correct? Select all correct answers. a. 1 mole of NI3 decomposes to produce 0.5 moles of N2 and 1.5 moles of I2 b. 2 moles of NI3 decomposes to produce 1 mole of N2 and 3 moles of I2. c. ...
Physical Limits of Computing
... amazing gift, in the form of the very sophisticated modern understanding of physics, as embodied in the Standard Model of particle physics. According to all available evidence, this model explains the world so successfully that apparently no known phenomenon fails to be encompassed within it. That ...
... amazing gift, in the form of the very sophisticated modern understanding of physics, as embodied in the Standard Model of particle physics. According to all available evidence, this model explains the world so successfully that apparently no known phenomenon fails to be encompassed within it. That ...
Dissipative particle dynamics with energy conservation
... be even functions under time-reversal.13 3. Since the thermodynamic forces change their sign under the exchange i ] j, then L (p) and L (q) must be invariant under ij ij this transformation. This fact is crucial in the derivation of the transport properties of the system. In the linear non-equilibri ...
... be even functions under time-reversal.13 3. Since the thermodynamic forces change their sign under the exchange i ] j, then L (p) and L (q) must be invariant under ij ij this transformation. This fact is crucial in the derivation of the transport properties of the system. In the linear non-equilibri ...
Lehninger Principles of Biochemistry Fourth Edition David L. Nelson
... found on the Earth today. About four billion years ago, ...
... found on the Earth today. About four billion years ago, ...
1994 Released Exam
... Directions: Each set of letteredchoicesbelow refers to the numberedquestionsor statementsimmediately following it. Select the one letteredchoice that bestanswerseach questionor bestfits each statementand then fill in the correspondingoval on the answersheet.A choice may be used once, more than once, ...
... Directions: Each set of letteredchoicesbelow refers to the numberedquestionsor statementsimmediately following it. Select the one letteredchoice that bestanswerseach questionor bestfits each statementand then fill in the correspondingoval on the answersheet.A choice may be used once, more than once, ...
Measurements - Effingham County Schools
... Chemical Properties/Chemical Changes Chemical property relates to a substance’s ability to ...
... Chemical Properties/Chemical Changes Chemical property relates to a substance’s ability to ...
Material Safety Data Sheet
... The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should ...
... The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should ...
Chapter 11: Reactions of Alkyl Halides There are two basic types of
... doubles. Halve the concentration of one and the rate is halved. Rate = k [RX][Nuc] Concentration of both species affects the number of times collisions can occur. The more times they can collide, the more times they can react. For the reaction to occur, two things have to fall into place: • Need eno ...
... doubles. Halve the concentration of one and the rate is halved. Rate = k [RX][Nuc] Concentration of both species affects the number of times collisions can occur. The more times they can collide, the more times they can react. For the reaction to occur, two things have to fall into place: • Need eno ...
ANSWER KEY Chemistry CPA Final Exam Study Guide Final Exam
... Unit 8: Gas Laws and KMT Kinetic Molecular Theory and Heating Curves 38. What is the kinetic molecular theory? Gas particles are always in constant, random motion. The higher the temperature, the higher the kinetic energy. Gas particles collide with one another in perfectly elastic collisions. As te ...
... Unit 8: Gas Laws and KMT Kinetic Molecular Theory and Heating Curves 38. What is the kinetic molecular theory? Gas particles are always in constant, random motion. The higher the temperature, the higher the kinetic energy. Gas particles collide with one another in perfectly elastic collisions. As te ...
Book-Abstracts - The Fritz Haber Center for Molecular dynamics
... The dissociation of oxygen on a clean aluminum surface is studied theoretically. A non adiabatic quantum dynamical model is used, based on four electronically distinct potential energy surfaces characterized by the extent of charge transfer from the metal to the adsorbate. A flat surface approximati ...
... The dissociation of oxygen on a clean aluminum surface is studied theoretically. A non adiabatic quantum dynamical model is used, based on four electronically distinct potential energy surfaces characterized by the extent of charge transfer from the metal to the adsorbate. A flat surface approximati ...
Export To Word
... structures. Repeating (periodic) patterns of physical and chemical properties occur among elements that define groups of elements with similar properties. The periodic table displays the repeating patterns, which are related to the atom's outermost electrons. Atoms bond with each other to form compo ...
... structures. Repeating (periodic) patterns of physical and chemical properties occur among elements that define groups of elements with similar properties. The periodic table displays the repeating patterns, which are related to the atom's outermost electrons. Atoms bond with each other to form compo ...
Example 1: An experiment shows that 64g of
... Calculate n in the formula BaCl2.nH2O 2. A sample of magnesium sulphate crystals weighing 0.942 g was heated to drive off the water of crystallization. When it reached constant mass, the mass of the residue was 0. 461g. Calculate the empirical formula of the hydrate 3. A sample of calcium sulphate c ...
... Calculate n in the formula BaCl2.nH2O 2. A sample of magnesium sulphate crystals weighing 0.942 g was heated to drive off the water of crystallization. When it reached constant mass, the mass of the residue was 0. 461g. Calculate the empirical formula of the hydrate 3. A sample of calcium sulphate c ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.