AP Chemistry:
... This Practice Exam from the 2013 international administration is provided by the College Board for AP Exam preparation. Teachers are permitted to download the materials and make copies to use with their students in a classroom setting only. To maintain the security of this exam, teachers should coll ...
... This Practice Exam from the 2013 international administration is provided by the College Board for AP Exam preparation. Teachers are permitted to download the materials and make copies to use with their students in a classroom setting only. To maintain the security of this exam, teachers should coll ...
DEPARTMENT OF CHEMISTRY, CFS, IIUM
... variety of matter is recognized is called a property. A characteristic that depends upon the amount of matter in the sample is called an extensive property. A characteristic that does not depend upon the amount of matter is called an intensive property. A characteristic that can be observed without ...
... variety of matter is recognized is called a property. A characteristic that depends upon the amount of matter in the sample is called an extensive property. A characteristic that does not depend upon the amount of matter is called an intensive property. A characteristic that can be observed without ...
Equilibrium and Kinetic Studies of Ligand
... buffer or bis-tris (bis-[2-hydroxyethyl]iminotris [hydroxy ethyl]methane) buffer are used (9). The chromatograms of other metal-HQS complexes exhibited single peaks even when the eluents contained a phosphate buffer. We have shown that this anomalous behavior of the alu minum complex can be explain ...
... buffer or bis-tris (bis-[2-hydroxyethyl]iminotris [hydroxy ethyl]methane) buffer are used (9). The chromatograms of other metal-HQS complexes exhibited single peaks even when the eluents contained a phosphate buffer. We have shown that this anomalous behavior of the alu minum complex can be explain ...
Document
... addition of FBSM to chalcones using cinchona-based phase transfer catalysts. Michael addition between nitromethane and chalcone with high ee and chemical yields using cinchona alkaloid-derived chiral bifunctional thiourea asan effective organocatalyst. ...
... addition of FBSM to chalcones using cinchona-based phase transfer catalysts. Michael addition between nitromethane and chalcone with high ee and chemical yields using cinchona alkaloid-derived chiral bifunctional thiourea asan effective organocatalyst. ...
Kinetics lecture
... • In other words, there is a minimum amount of energy required for reaction: the activation energy, Ea. • Just as a ball cannot get over a hill if it does not roll up the hill with enough energy, a reaction cannot occur unless the molecules possess sufficient energy to get over the ...
... • In other words, there is a minimum amount of energy required for reaction: the activation energy, Ea. • Just as a ball cannot get over a hill if it does not roll up the hill with enough energy, a reaction cannot occur unless the molecules possess sufficient energy to get over the ...
Ionic contamination testing in a no
... contamination testing and they can be measured. Due to the fact that these ions are not present on the board as such, but only in extraction medium, and the fact that the ionic contamination test does not provide any information about the nature of the ions, it does not give any information about th ...
... contamination testing and they can be measured. Due to the fact that these ions are not present on the board as such, but only in extraction medium, and the fact that the ionic contamination test does not provide any information about the nature of the ions, it does not give any information about th ...
ExamView - Quiz 3--Heat and Thermo PRACTICE.tst
... 30. Which of the following is a way to improve the efficiency of a heat engine? a. increase Q h b. reduce Q h c. reduce W net d. increase Q c 31. An ideal heat engine has an efficiency of 50 percent. Which of the following statements is not true? a. The amount of energy exhausted as heat equals the ...
... 30. Which of the following is a way to improve the efficiency of a heat engine? a. increase Q h b. reduce Q h c. reduce W net d. increase Q c 31. An ideal heat engine has an efficiency of 50 percent. Which of the following statements is not true? a. The amount of energy exhausted as heat equals the ...
Fragilities of Liquids Predicted from the Random First Order
... for the slowing of rates, nonexponential relaxations in the super cooled liquid state and the behavior of thermodynamic properties on cooling(1). Quantitative differences in behavior of different substances sometimes obscure this universality. This has led to a classification of liquids into “fragil ...
... for the slowing of rates, nonexponential relaxations in the super cooled liquid state and the behavior of thermodynamic properties on cooling(1). Quantitative differences in behavior of different substances sometimes obscure this universality. This has led to a classification of liquids into “fragil ...
Chemistry Spell check on
... to the following equation. 2H2O2(aq) → 2H2O(ℓ) + O2(g) (a) At room temperature, the reaction is very slow. It can be speeded up by heating the reaction mixture. State why increasing the temperature causes an increase in reaction rate. ...
... to the following equation. 2H2O2(aq) → 2H2O(ℓ) + O2(g) (a) At room temperature, the reaction is very slow. It can be speeded up by heating the reaction mixture. State why increasing the temperature causes an increase in reaction rate. ...
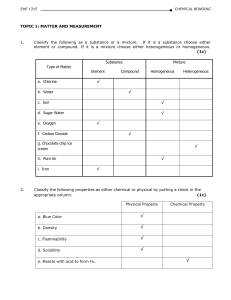
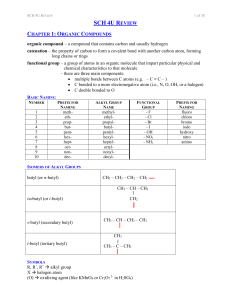
SCH 4U REVIEW Notes
... polymer – a molecule of large molar mass that consists of many repeating subunits called monomers; two types: addition and condensation monomer – a molecule or compound usually containing carbon and of relatively low molecular weight and simple structure which is capable of conversion to polymers by ...
... polymer – a molecule of large molar mass that consists of many repeating subunits called monomers; two types: addition and condensation monomer – a molecule or compound usually containing carbon and of relatively low molecular weight and simple structure which is capable of conversion to polymers by ...
KINETICS questions
... (b) Calculate the value of the specific rate constant k at 30C and specify its units. (c) Calculate the value of the initial rate of this reaction at 30C for the initial concentrations shown in experiment 6. (d) Assume that the reaction goes to completion. Under the conditions specified for experi ...
... (b) Calculate the value of the specific rate constant k at 30C and specify its units. (c) Calculate the value of the initial rate of this reaction at 30C for the initial concentrations shown in experiment 6. (d) Assume that the reaction goes to completion. Under the conditions specified for experi ...
Document
... to the following equation. 2H2O2(aq) → 2H2O(ℓ) + O2(g) (a) At room temperature, the reaction is very slow. It can be speeded up by heating the reaction mixture. State why increasing the temperature causes an increase in reaction rate. ...
... to the following equation. 2H2O2(aq) → 2H2O(ℓ) + O2(g) (a) At room temperature, the reaction is very slow. It can be speeded up by heating the reaction mixture. State why increasing the temperature causes an increase in reaction rate. ...
Chemistry
... NGSS implementation are great, and revision of curriculum requires careful consideration of these changes as well as time to develop, pilot, and implement. During this transitional period, resources in suppo ...
... NGSS implementation are great, and revision of curriculum requires careful consideration of these changes as well as time to develop, pilot, and implement. During this transitional period, resources in suppo ...
Fall 2012
... at 10,000 m where the temperature is expected to be -10.3 °C and the pressure will be around 420 torr. What is the maximum volume of helium that the meteorologist can put in the balloon at 723.1 torr and 27.3 °C (conditions on the earth's surface) that will allow cruising at 10,000 m without exceedi ...
... at 10,000 m where the temperature is expected to be -10.3 °C and the pressure will be around 420 torr. What is the maximum volume of helium that the meteorologist can put in the balloon at 723.1 torr and 27.3 °C (conditions on the earth's surface) that will allow cruising at 10,000 m without exceedi ...
Dr David`s Chemistry Revision Themes
... A reaction mixture comprising gaseous reactants and products is contained in a cylinder fitted with a movable piston. Pressure is applied to the piston and the reaction mixture compressed into a smaller volume. Assuming the temperature remains constant, what effect will this have on the rate of reac ...
... A reaction mixture comprising gaseous reactants and products is contained in a cylinder fitted with a movable piston. Pressure is applied to the piston and the reaction mixture compressed into a smaller volume. Assuming the temperature remains constant, what effect will this have on the rate of reac ...
3.8 Useful Relationships - Molecular Diversity Preservation
... a system depends on the inherent qualities, or properties, of the materials in the system, such as composition and physical form, as well as the environmental variables (temperature, pressure, electric field, magnetic field, etc.). Internal energy can have many forms, including, sensible and latent ...
... a system depends on the inherent qualities, or properties, of the materials in the system, such as composition and physical form, as well as the environmental variables (temperature, pressure, electric field, magnetic field, etc.). Internal energy can have many forms, including, sensible and latent ...
Thermodynamics & Statistical Mechanics:
... one percentage point, and only a thousand people were polled, then I know the result is statistically meaningless. We can easily appreciate that if we do statistics on a thermodynamic system containing 1024 particles then we are going to obtain results which are valid to incredible accuracy. In fact ...
... one percentage point, and only a thousand people were polled, then I know the result is statistically meaningless. We can easily appreciate that if we do statistics on a thermodynamic system containing 1024 particles then we are going to obtain results which are valid to incredible accuracy. In fact ...
Chemistry Entrance Material for Grade 11 to 12 Answer Key
... [-C-] Can bubbles of vapour form anywhere within the liquid? Yes Know when a liquid boils 19. In general, a liquid boils when [-A-] its vapour pressure is 1 atmosphere [-B-] its vapour pressure is 760 mm Hg [-C-] its temperature is 100oC [-D-] its vapour pressure equals the surrounding pressure [-E- ...
... [-C-] Can bubbles of vapour form anywhere within the liquid? Yes Know when a liquid boils 19. In general, a liquid boils when [-A-] its vapour pressure is 1 atmosphere [-B-] its vapour pressure is 760 mm Hg [-C-] its temperature is 100oC [-D-] its vapour pressure equals the surrounding pressure [-E- ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.