Heat Chapter 12: Thermodynamics
... process can naturally or spontaneously take place. • Heat does not flow spontaneously from a colder to a warmer body. • In a thermal cycle, heat energy cannot be completely transformed into mechanical work. • The total entropy of the universe increases in every natural process. The Third Law of Ther ...
... process can naturally or spontaneously take place. • Heat does not flow spontaneously from a colder to a warmer body. • In a thermal cycle, heat energy cannot be completely transformed into mechanical work. • The total entropy of the universe increases in every natural process. The Third Law of Ther ...
CHAPTER 2: THE ATOMS AND MOLECULES OF ANCIENT EARTH
... 1. H = difference in potential energy between reactants and products a. Exothermic reactions—Potential energy of products is less than that of reactants. (1) Heat is given off during the reaction. (2) H = (–). b. Endothermic reactions—Potential energy of products is greater than that of reactants. ...
... 1. H = difference in potential energy between reactants and products a. Exothermic reactions—Potential energy of products is less than that of reactants. (1) Heat is given off during the reaction. (2) H = (–). b. Endothermic reactions—Potential energy of products is greater than that of reactants. ...
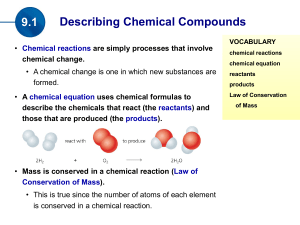
(the products). Mass is conserved in a chemical reaction
... see, hear, feel, smell, etc.? • Using your clues, determine if a physical or chemical change is taking place when ...
... see, hear, feel, smell, etc.? • Using your clues, determine if a physical or chemical change is taking place when ...
Chemistry Standard Outline
... Chemical Reactions, Law of Conservation of Matter and Heat SC2 Students will relate how the Law of Conservation of Matter is used to determine chemical composition in compounds and chemical reactions. SC2a. Identify and balance the following types of chemical equations: • Synthesis • Decomposition • ...
... Chemical Reactions, Law of Conservation of Matter and Heat SC2 Students will relate how the Law of Conservation of Matter is used to determine chemical composition in compounds and chemical reactions. SC2a. Identify and balance the following types of chemical equations: • Synthesis • Decomposition • ...
P2-Equilibrium Activity
... Not all chemical reactions reach completion where the limiting reactant is consumed completely. In fact, most chemical reactions that occur in living systems never reach completion. Rather, they produce some amount of product then appear to stop reacting in the forward direction, never fully consumi ...
... Not all chemical reactions reach completion where the limiting reactant is consumed completely. In fact, most chemical reactions that occur in living systems never reach completion. Rather, they produce some amount of product then appear to stop reacting in the forward direction, never fully consumi ...
Chemistry: Introduction to Chemical Reactions Guided Inquiry What
... Elements and/or compounds (called reactants) are changed to create one or more new substances (called products). If new compounds aren’t formed then no reaction has occurred. ...
... Elements and/or compounds (called reactants) are changed to create one or more new substances (called products). If new compounds aren’t formed then no reaction has occurred. ...
D12E12Safety1\4Curr\emet
... 7.2.1 state that “internal” or “intrinsic” energy (U) is related to the motions of the molecules of a substance or a system 7.2.2 state that internal energy is derived only from molecular motions and vibrations, is dependent only on thermodynamic temperature and is energy stored in the molecules 7.2 ...
... 7.2.1 state that “internal” or “intrinsic” energy (U) is related to the motions of the molecules of a substance or a system 7.2.2 state that internal energy is derived only from molecular motions and vibrations, is dependent only on thermodynamic temperature and is energy stored in the molecules 7.2 ...
Document
... CHEMICAL BONDS Bond formation involves the electrons (e-) in the outermost (valence) shell. A complete outer shell consists of 8 valence electrons (except H and He which have 2) Destruction of a bond corresponds to a release of energy. Generally double or triple bond energies are higher than for si ...
... CHEMICAL BONDS Bond formation involves the electrons (e-) in the outermost (valence) shell. A complete outer shell consists of 8 valence electrons (except H and He which have 2) Destruction of a bond corresponds to a release of energy. Generally double or triple bond energies are higher than for si ...
New Title
... 4. The substances you have at the beginning of a chemical reaction are called the 5. The substances you have when a chemical reaction is complete are called the 6. What do you read the arrow in a chemical equation as meaning? 7. Label each formula in the chemical equation below as either a reactant ...
... 4. The substances you have at the beginning of a chemical reaction are called the 5. The substances you have when a chemical reaction is complete are called the 6. What do you read the arrow in a chemical equation as meaning? 7. Label each formula in the chemical equation below as either a reactant ...
State of the system
... State of the system q A certain number of variables specify the state of the system. (a) intensive variables – independent of the size of the system (density, dielectric constant, molar free energy, chemical potential, pressure, specific heat and temperature) (b) extensive variables – dependent on ...
... State of the system q A certain number of variables specify the state of the system. (a) intensive variables – independent of the size of the system (density, dielectric constant, molar free energy, chemical potential, pressure, specific heat and temperature) (b) extensive variables – dependent on ...
Matter 1. ______ is anything that has ______ and takes up ______
... 7. Classification of Matter – matter can be classified by its physical and chemical properties. a _______________ _________________– a characteristic of a substance that can be observed without changing the substance into another substance. Examples: physical state (solid, liquid, gas) electrical an ...
... 7. Classification of Matter – matter can be classified by its physical and chemical properties. a _______________ _________________– a characteristic of a substance that can be observed without changing the substance into another substance. Examples: physical state (solid, liquid, gas) electrical an ...
Chemistry Test Review - Greenslime Home Page
... What is the difference between physical properties, physical changes & chemical changes? a. Physical properties are what you see, feel hear from objects and can be used to describe it. b. Physical changes occur when you alter the shape or size of an object, but it is still made of the same “stuff” a ...
... What is the difference between physical properties, physical changes & chemical changes? a. Physical properties are what you see, feel hear from objects and can be used to describe it. b. Physical changes occur when you alter the shape or size of an object, but it is still made of the same “stuff” a ...
Chapter 1 Chemistry: The Study of Matter
... Another name for chemical change When one or more substances are changed into new substances. Reactants- stuff you start with Products- What you make NEW PROPERTIES Because each substance has its own properties ...
... Another name for chemical change When one or more substances are changed into new substances. Reactants- stuff you start with Products- What you make NEW PROPERTIES Because each substance has its own properties ...
Thermochemistry
... reactions and energy changes involving heat. • Energy: The capacity to do work or transfer heat. • Work: Energy used to move an object with mass against a force. W = F x d • Heat: The energy transferred from a hotter object to a colder one. • The unit of energy is a Joule. 1J=1kg-m2/s2 ...
... reactions and energy changes involving heat. • Energy: The capacity to do work or transfer heat. • Work: Energy used to move an object with mass against a force. W = F x d • Heat: The energy transferred from a hotter object to a colder one. • The unit of energy is a Joule. 1J=1kg-m2/s2 ...
Matter- Types and Changes
... • CO2 contains 1 atom of carbon and two atoms of oxygen all chemically linked. • H2SO4 contains 2 hydrogen, 1 sulfur, and 4 oxygen atoms. • (NH4)2C2O4 - A subscript outside parentheses applies to everything within the parentheses; 2 N, 8 H, 2 C, 4 O ...
... • CO2 contains 1 atom of carbon and two atoms of oxygen all chemically linked. • H2SO4 contains 2 hydrogen, 1 sulfur, and 4 oxygen atoms. • (NH4)2C2O4 - A subscript outside parentheses applies to everything within the parentheses; 2 N, 8 H, 2 C, 4 O ...
3 - Zheng Research Group
... Uneven distribution of oil reserve globally: >60% stored in Persian gulf area. The global fossil fuel reserve is decreasing very fast in recent decades. 14 ...
... Uneven distribution of oil reserve globally: >60% stored in Persian gulf area. The global fossil fuel reserve is decreasing very fast in recent decades. 14 ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.