Chem 2A Final Review
... 58. Both water and sulfur dioxide are produced from the reaction of sulfuric acid (H 2SO4) with copper metal in the following balanced equation. How many moles of H2O will be produced at the same time that 10.0 moles of SO2 is produced? 2H2SO4 + Cu SO2 + 2H2O + CuSO4 ...
... 58. Both water and sulfur dioxide are produced from the reaction of sulfuric acid (H 2SO4) with copper metal in the following balanced equation. How many moles of H2O will be produced at the same time that 10.0 moles of SO2 is produced? 2H2SO4 + Cu SO2 + 2H2O + CuSO4 ...
Quarter 1
... C5.2C Draw pictures to distinguish the relationships between atoms in physical and chemical changes. ...
... C5.2C Draw pictures to distinguish the relationships between atoms in physical and chemical changes. ...
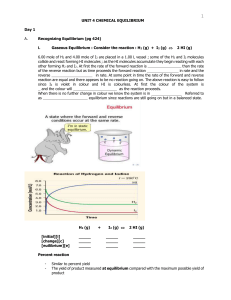
Unit 4 - Chemical Equilibrium
... _ _ _ _ _ _ _ _ _ _. This means the products can react together and turn back into the _ _ _ _ _ _ _ _ reactants. In other words, the reaction can go _ _ _ _ ways. When a reversible reaction is set up in a _ _ _ _ _ _ container, the forward reaction happens much faster than the reverse reaction at f ...
... _ _ _ _ _ _ _ _ _ _. This means the products can react together and turn back into the _ _ _ _ _ _ _ _ reactants. In other words, the reaction can go _ _ _ _ ways. When a reversible reaction is set up in a _ _ _ _ _ _ container, the forward reaction happens much faster than the reverse reaction at f ...
ESO - ENCIGA
... about them and to formulate laws and principles based on these facts. The organized knowledge that is derived from scientific studies is continuously tested by subsequent investigation and can be modified by its results. Science does not give statements of absolute eternal truth, it only provides th ...
... about them and to formulate laws and principles based on these facts. The organized knowledge that is derived from scientific studies is continuously tested by subsequent investigation and can be modified by its results. Science does not give statements of absolute eternal truth, it only provides th ...
9.2 Oxidation Numbers
... and coke (a carbon‑rich mixture produced by heating coal). This method for isolating phosphorus, called the furnace process, is summarized in the first equation below. The other equations show how phosphorus can be converted into ammonium phosphate. 2Ca3(PO4)2 + 6SiO2 + 10C → P4 + 10CO + 6CaSiO3 P4 ...
... and coke (a carbon‑rich mixture produced by heating coal). This method for isolating phosphorus, called the furnace process, is summarized in the first equation below. The other equations show how phosphorus can be converted into ammonium phosphate. 2Ca3(PO4)2 + 6SiO2 + 10C → P4 + 10CO + 6CaSiO3 P4 ...
CHE 110 Dr. Nicholas Bizier Office DS 337b email
... Alcohol has a density of 0.76 g/ml. How many grams of alcohol would it take to fill a 2 fluid ounce shot glass? ...
... Alcohol has a density of 0.76 g/ml. How many grams of alcohol would it take to fill a 2 fluid ounce shot glass? ...
35 IChO Problems 1-13
... has a two-fold degeneracy except for the zero level which is nondegenerate. Assuming a mean bond length between carbon atoms equal to 1.40 Å, determine the wavelength of the lowest electronic transition. ...
... has a two-fold degeneracy except for the zero level which is nondegenerate. Assuming a mean bond length between carbon atoms equal to 1.40 Å, determine the wavelength of the lowest electronic transition. ...
chemical kinetics - Berkeley City College
... k1 Step-1: NO2 + NO2 > NO3 + NO; [slow; rate-determining] Step-2: NO3 + CO > CO2 + NO; [fast] The rate law for the rate-determining step: Rate = k1[NO2]2, which is identical in form to the rate law obtained experimentally, in which k1 = k. The second step, which occurs very fast, does not influe ...
... k1 Step-1: NO2 + NO2 > NO3 + NO; [slow; rate-determining] Step-2: NO3 + CO > CO2 + NO; [fast] The rate law for the rate-determining step: Rate = k1[NO2]2, which is identical in form to the rate law obtained experimentally, in which k1 = k. The second step, which occurs very fast, does not influe ...
Chem 11 Stoichiometry (mol-mol) Using the formulas we have
... Using the coefficients as moles, we can determine the mass of the each of the reactants present and also for the product formed. Mass of Reactants: m = nxM = (1 mol N2)(28.0134g/mol N2) = 28.0134g N2 m = nxM = (3 mol H2)(2.01588g/mol H2) = 6.04764g H2 Total mass of Reactants = 28.0134g N2 + 6.04764g ...
... Using the coefficients as moles, we can determine the mass of the each of the reactants present and also for the product formed. Mass of Reactants: m = nxM = (1 mol N2)(28.0134g/mol N2) = 28.0134g N2 m = nxM = (3 mol H2)(2.01588g/mol H2) = 6.04764g H2 Total mass of Reactants = 28.0134g N2 + 6.04764g ...
Document
... Any property that only depends on object’s current state or condition Independence from method, path or mechanism by which change occurs is important feature of all state functions Some State functions, E, P, t, and V : ...
... Any property that only depends on object’s current state or condition Independence from method, path or mechanism by which change occurs is important feature of all state functions Some State functions, E, P, t, and V : ...
Chapter 8 and 9
... Alcohol has a density of 0.76 g/ml. How many grams of alcohol would it take to fill a 2 fluid ounce shot glass? ...
... Alcohol has a density of 0.76 g/ml. How many grams of alcohol would it take to fill a 2 fluid ounce shot glass? ...
chemistry - Ethiopian Ministry of Education
... more closely at nature’s way of working. Understanding change is closely related to understanding the nature and composition of matter- the physical material of the universe. Matter is anything that occupies space and has mass. It has long been known that matter can change or be made to change from ...
... more closely at nature’s way of working. Understanding change is closely related to understanding the nature and composition of matter- the physical material of the universe. Matter is anything that occupies space and has mass. It has long been known that matter can change or be made to change from ...
Water Vapor and Mechanical Work: A Comparison of
... Emanuel 1998; Emanuel 2003). It is argued here that, although many atmospheric phenomena can be viewed at least in part as heat engines, the analogy with a Carnot cycle often overestimates the conversion of internal energy into kinetic energy in the presence of water vapor. To better assess the role ...
... Emanuel 1998; Emanuel 2003). It is argued here that, although many atmospheric phenomena can be viewed at least in part as heat engines, the analogy with a Carnot cycle often overestimates the conversion of internal energy into kinetic energy in the presence of water vapor. To better assess the role ...
Ch 10 Practice Problems 1. Consider the process A(l) A(s). Which
... B) equal to zero. C) greater than zero. D) More information is needed. q is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. H is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. E is A) less than zero. B) equal to z ...
... B) equal to zero. C) greater than zero. D) More information is needed. q is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. H is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. E is A) less than zero. B) equal to z ...
Unit 8: Reactions
... Objective: The amount of matter in reactants equals that in products! The mass on the reactants (left) side of the arrow and the mass on the products (right) side of the arrow MUST equal each other as the Law of Conservation of Mass states that mass may not be created or destroyed in any chemical re ...
... Objective: The amount of matter in reactants equals that in products! The mass on the reactants (left) side of the arrow and the mass on the products (right) side of the arrow MUST equal each other as the Law of Conservation of Mass states that mass may not be created or destroyed in any chemical re ...
Chem 1B Fa2015 FinalExam Review
... [Ni(NH3)2Cl2] is a tetrahedral complex, which is a weak-field complex, and with 3d8 electron configuration for Ni2+, the complex [Ni(NH3)2Cl2] would be paramagnetic. In addition, a tetrahedral complex [Ni(NH3)2Cl2] will not exhibit isomerism. (Show d8 configuration in tetrahedral crystal field diagr ...
... [Ni(NH3)2Cl2] is a tetrahedral complex, which is a weak-field complex, and with 3d8 electron configuration for Ni2+, the complex [Ni(NH3)2Cl2] would be paramagnetic. In addition, a tetrahedral complex [Ni(NH3)2Cl2] will not exhibit isomerism. (Show d8 configuration in tetrahedral crystal field diagr ...
Synthetic Polymers - McQuarrie General Chemistry
... can be increased by increasing the number of crosslinks between chains. High elasticity is found in substances composed of long polymer chains joined by sparsely distributed cross-links, such as the polymer chains found in rubber bands. Natural rubber is composed of chains of cis-1,4-isoprene units ...
... can be increased by increasing the number of crosslinks between chains. High elasticity is found in substances composed of long polymer chains joined by sparsely distributed cross-links, such as the polymer chains found in rubber bands. Natural rubber is composed of chains of cis-1,4-isoprene units ...
CHE-310 Organic Chemistry I_
... For alkyl halides, alcohols and ethers, be able to name compounds correctly (nomenclature). Where necessay, be able to specify congiguration in the name. Know the two new mechanisms that we have learned in these chapters: SN2, SN1. Know which mechanisms go with which reactions under which conditions ...
... For alkyl halides, alcohols and ethers, be able to name compounds correctly (nomenclature). Where necessay, be able to specify congiguration in the name. Know the two new mechanisms that we have learned in these chapters: SN2, SN1. Know which mechanisms go with which reactions under which conditions ...
Phase Stability of the Earth-Abundant Tin
... Centre for Sustainable Chemical Technologies, Department of Chemistry, University of Bath, Bath, U.K. ABSTRACT: The various phases of tin sulfide have been studied as semiconductors since the 1960s and are now being investigated as potential earth-abundant photovoltaic and photocatalytic materials. O ...
... Centre for Sustainable Chemical Technologies, Department of Chemistry, University of Bath, Bath, U.K. ABSTRACT: The various phases of tin sulfide have been studied as semiconductors since the 1960s and are now being investigated as potential earth-abundant photovoltaic and photocatalytic materials. O ...
Sample Chapter - Chapter 4
... and is actually somewhat misleading, because it shows all the reactants and products as if they were intact, undissociated compounds: ...
... and is actually somewhat misleading, because it shows all the reactants and products as if they were intact, undissociated compounds: ...
Theories of the constitution of gases in the early nineteenth century
... it gave out in chemical reaction were the same – both contained in the caloric flesh of the atom – was one of the most misleading ideas in the chemistry of the period. Perhaps the reason for this mistake was the lack of interest in affinity, of which heat of reaction was the most obvious measure. Th ...
... it gave out in chemical reaction were the same – both contained in the caloric flesh of the atom – was one of the most misleading ideas in the chemistry of the period. Perhaps the reason for this mistake was the lack of interest in affinity, of which heat of reaction was the most obvious measure. Th ...
Fundamentals of Combustion
... 2.3.1.1 Binary mixtures . . . . . . . . . . . 2.3.1.2 Entropy of mixing . . . . . . . . . . 2.3.1.3 Mixtures of constant mass fraction . 2.3.2 Summary of properties for the Dalton mixture 2.3.2.1 Mass basis . . . . . . . . . . . . . . . 2.3.2.2 Molar basis . . . . . . . . . . . . . . 2.3.3 Amagat mo ...
... 2.3.1.1 Binary mixtures . . . . . . . . . . . 2.3.1.2 Entropy of mixing . . . . . . . . . . 2.3.1.3 Mixtures of constant mass fraction . 2.3.2 Summary of properties for the Dalton mixture 2.3.2.1 Mass basis . . . . . . . . . . . . . . . 2.3.2.2 Molar basis . . . . . . . . . . . . . . 2.3.3 Amagat mo ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.