Description: This is an advanced placement course designed to
... 2. First law: change in enthalpy; heat of formation; heat of reaction; Hess's law; heats of vaporization and fusion; calorimetry 3. Second law: entropy; free energy of formation; free energy of reaction; dependence of change in free energy on enthalpy and entropy changes 4. Relationship of change in ...
... 2. First law: change in enthalpy; heat of formation; heat of reaction; Hess's law; heats of vaporization and fusion; calorimetry 3. Second law: entropy; free energy of formation; free energy of reaction; dependence of change in free energy on enthalpy and entropy changes 4. Relationship of change in ...
SF Chemical Kinetics Michaelmas 2011 L5
... from first principles using fundamental physics. Any microscopic level theory of chemical reaction kinetics must result in the derivation of an expression for the rate constant that is consistent with the empirical Arrhenius equation. A microscopic model should furthermore provide a reasonable inter ...
... from first principles using fundamental physics. Any microscopic level theory of chemical reaction kinetics must result in the derivation of an expression for the rate constant that is consistent with the empirical Arrhenius equation. A microscopic model should furthermore provide a reasonable inter ...
Thermochemistry
... standard enthalpy changes for many reactions. In an application of Hess’s Law, it is as if the reactants are decomposed into their elements, and then the elements are recombined into the desired products. Since enthalpies of reaction are independent of pathway, this provides an accurate way to calcu ...
... standard enthalpy changes for many reactions. In an application of Hess’s Law, it is as if the reactants are decomposed into their elements, and then the elements are recombined into the desired products. Since enthalpies of reaction are independent of pathway, this provides an accurate way to calcu ...
PPT
... • First, we’ll figure out where vapor, liquid and ice are in equilibrium, and the energy changes associated with the transitions – Can calculate changes such as heat released so we can treat the energy changes in our parcel – Can also figure out if the change is favored (e.g., if the transition is a ...
... • First, we’ll figure out where vapor, liquid and ice are in equilibrium, and the energy changes associated with the transitions – Can calculate changes such as heat released so we can treat the energy changes in our parcel – Can also figure out if the change is favored (e.g., if the transition is a ...
Inweld Nibral, Aluminum Bronze 46
... Description and Applications: Inweld Nibral is sometimes referred to its generic name, Nickel-Aluminum-Bronze. This filler metal is used for MIG and TIG welding of cast and wrought nickel-aluminum-bronze parts where there is a requirement for high resistance to corrosion, erosion and cavitation in s ...
... Description and Applications: Inweld Nibral is sometimes referred to its generic name, Nickel-Aluminum-Bronze. This filler metal is used for MIG and TIG welding of cast and wrought nickel-aluminum-bronze parts where there is a requirement for high resistance to corrosion, erosion and cavitation in s ...
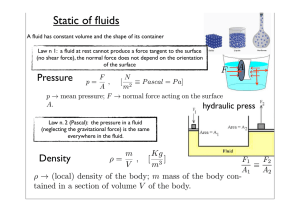
Static of fluids
... Second principle of thermodynamics It is impossible to realize a cyclic machine whose only result is to transform in work all the heat absorbed by a homogeneous source (Kelvin-Planck formulation). ...
... Second principle of thermodynamics It is impossible to realize a cyclic machine whose only result is to transform in work all the heat absorbed by a homogeneous source (Kelvin-Planck formulation). ...
Chemical Equations
... “bookkeeping” technique designed to count the number of each type of atom (ion) represented on each side of a chemical equation d) ________________________ = whole number that appears to the right and below a chemical symbol in a chemical formula (if no number is written it is assumed to be “1”) ...
... “bookkeeping” technique designed to count the number of each type of atom (ion) represented on each side of a chemical equation d) ________________________ = whole number that appears to the right and below a chemical symbol in a chemical formula (if no number is written it is assumed to be “1”) ...
Review - UMD Physics
... 1. (4 pts) A 200 g block of copper at a temperature of 55 oC is put into an insulated beaker of water at 20 oC. The two come to thermal equilibrium at a temperature of about 30 oC – much closer to the original temperature of the water than of the copper. (From the macroscopic point of view, the spec ...
... 1. (4 pts) A 200 g block of copper at a temperature of 55 oC is put into an insulated beaker of water at 20 oC. The two come to thermal equilibrium at a temperature of about 30 oC – much closer to the original temperature of the water than of the copper. (From the macroscopic point of view, the spec ...
v = Y
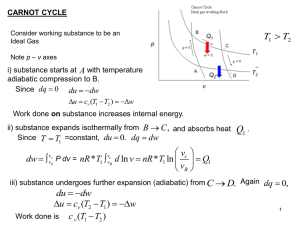
... reservoir when heat is discarded into it (TC). ◦ Any finite temperature drop would result in an irreversible processes. ◦ Every process that involves heat transfer must be isothermal. ◦ Any process in which the the working substance is between TH and TC, there must be no heat transfer into the hot o ...
... reservoir when heat is discarded into it (TC). ◦ Any finite temperature drop would result in an irreversible processes. ◦ Every process that involves heat transfer must be isothermal. ◦ Any process in which the the working substance is between TH and TC, there must be no heat transfer into the hot o ...
Final Exam Study Guide Word document
... In an electrochemical cell, oxidation occurs at which electrode? In an electrochemical process called "electrolysis", H2 gas and O2 gas can be obtained by passing an electric current through liquid water, 2H2O(l) --> 2H2(g) + O2(g). Which species is the OXIDIZING AGENT and which species is the REDUC ...
... In an electrochemical cell, oxidation occurs at which electrode? In an electrochemical process called "electrolysis", H2 gas and O2 gas can be obtained by passing an electric current through liquid water, 2H2O(l) --> 2H2(g) + O2(g). Which species is the OXIDIZING AGENT and which species is the REDUC ...
Atoms and Nuclei
... represent the three isotopes of hydrogen. All contain one proton (Z = 1 or it wouldn’t be hydrogen!) but these three isotopes contain zero, one, and two neutrons, respectively. The atomic number Z is sometimes included as a subscript but usually not since the chemical symbol implies the Z-value. The ...
... represent the three isotopes of hydrogen. All contain one proton (Z = 1 or it wouldn’t be hydrogen!) but these three isotopes contain zero, one, and two neutrons, respectively. The atomic number Z is sometimes included as a subscript but usually not since the chemical symbol implies the Z-value. The ...
20141113080528
... – The molar mass is the same as its atomic mass expressed in grams. (Ex: C is 12amu, so molar mass of C is 12 g) – A CO2 molecules is composed of one C atom (12 amu) and 2 O atoms (2 x 16amu = 32amu). So CO2 has a molar mass of 44 grams. (add the two amu ...
... – The molar mass is the same as its atomic mass expressed in grams. (Ex: C is 12amu, so molar mass of C is 12 g) – A CO2 molecules is composed of one C atom (12 amu) and 2 O atoms (2 x 16amu = 32amu). So CO2 has a molar mass of 44 grams. (add the two amu ...
Chemistry for Changing Times
... Groups of atoms chemically bonded together H represents a hydrogen atom H2 represents a hydrogen molecule How many atoms of O are in H2O2? Be careful when writing formulas for ...
... Groups of atoms chemically bonded together H represents a hydrogen atom H2 represents a hydrogen molecule How many atoms of O are in H2O2? Be careful when writing formulas for ...
Chapter 8 Thermochemistry
... • The standard molar enthalpy of formation, ∆Hfo, is equal to the enthalpy change • For one mole of a compound • At constant pressure of 1 atm • At a fixed temperature of 25 °C • From elements in their stable states at that temperature and pressure • Enthalpies of formation are tabulated in Table 8. ...
... • The standard molar enthalpy of formation, ∆Hfo, is equal to the enthalpy change • For one mole of a compound • At constant pressure of 1 atm • At a fixed temperature of 25 °C • From elements in their stable states at that temperature and pressure • Enthalpies of formation are tabulated in Table 8. ...
Lecture 5 Entropy
... If a closed system is not in a state of statistical equilibrium, its macroscopic state will vary in time, until ultimately the system reaches a state of maximum entropy. Moreover, at equilibrium, all microstates are equally probable. ...
... If a closed system is not in a state of statistical equilibrium, its macroscopic state will vary in time, until ultimately the system reaches a state of maximum entropy. Moreover, at equilibrium, all microstates are equally probable. ...
Matter - HCC Learning Web
... Potential & Kinetic Energy • Potential energy, PE, is stored energy; it results from position or composition. PE = mgh • Kinetic energy, KE, is the energy matter has as a result of motion. KE = ½ mv2 • Energy can be converted between the two types. • A boulder at the top of the hill has potential e ...
... Potential & Kinetic Energy • Potential energy, PE, is stored energy; it results from position or composition. PE = mgh • Kinetic energy, KE, is the energy matter has as a result of motion. KE = ½ mv2 • Energy can be converted between the two types. • A boulder at the top of the hill has potential e ...
FOUNTAIN UNIVERSITY, OSOGBO
... gas molecule; molecular theory of kinetic energy using the gas laws. Laws of thermodynamics: concepts of reversibility and entropy; zeroth, first, second and third laws of thermodynamics; relationships between Cp and Cv ; Gibbs energy; Phase equilibria: chemical equilibrium; activity of materials in ...
... gas molecule; molecular theory of kinetic energy using the gas laws. Laws of thermodynamics: concepts of reversibility and entropy; zeroth, first, second and third laws of thermodynamics; relationships between Cp and Cv ; Gibbs energy; Phase equilibria: chemical equilibrium; activity of materials in ...
HW 15 - Effingham County Schools
... 1. In a chemical change, the matter you start with is called the (reactant/product). 2. In a chemical change, the new matter is called the (reactant/product). 3. The state of matter where the molecules are packed tightly together is called (solid/gas). 4. The state of matter where the molecules have ...
... 1. In a chemical change, the matter you start with is called the (reactant/product). 2. In a chemical change, the new matter is called the (reactant/product). 3. The state of matter where the molecules are packed tightly together is called (solid/gas). 4. The state of matter where the molecules have ...
Eötvös Loránd Science University Faculty of Sciences Department of
... Knowledge of the material discussed at the lectures and the seminars. Solving assignments and writing midterm exams and the take-home exam. In case the student does not get or accept an offered final grade, an oral exam is necessary. ...
... Knowledge of the material discussed at the lectures and the seminars. Solving assignments and writing midterm exams and the take-home exam. In case the student does not get or accept an offered final grade, an oral exam is necessary. ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.