Figure 6.15 When a reaction
... Figure 6.9 When a system expands, it performs work on its surroundings by forcing all the molecules in another object in the surroundings to move in the same direction. Here we see the expansion of a gas raise a weight. The expansion of gases in the cylinders of automobiles do work by pushing on th ...
... Figure 6.9 When a system expands, it performs work on its surroundings by forcing all the molecules in another object in the surroundings to move in the same direction. Here we see the expansion of a gas raise a weight. The expansion of gases in the cylinders of automobiles do work by pushing on th ...
MSDS - Dudley Chemical Corporation
... representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the information must exercise their independent judgment in determining its appro ...
... representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the information must exercise their independent judgment in determining its appro ...
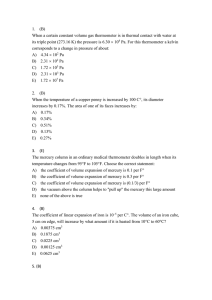
Quiz_MATH.rtf
... C) Q = (5/2)nRT, Eint = (5/2)nRT, W = 0 D) Q = (3/2)nRT, Eint = 0, W = –nRT E) Q = (5/2)nRT, Eint = (3/2)nRT, W = –nRT 17. (B) The temperature of n moles of an ideal monatomic gas is increased by T at constant volume. The energy Q absorbed as heat, change Eint in internal energy, and wo ...
... C) Q = (5/2)nRT, Eint = (5/2)nRT, W = 0 D) Q = (3/2)nRT, Eint = 0, W = –nRT E) Q = (5/2)nRT, Eint = (3/2)nRT, W = –nRT 17. (B) The temperature of n moles of an ideal monatomic gas is increased by T at constant volume. The energy Q absorbed as heat, change Eint in internal energy, and wo ...
All chemical reactions is change of electronic entanglement
... The essence of gravitational wave is electron, so that light-wave is a kind of gravitational wave full of energy. All the collection of mass can be considered as a set of energy or wave source . And the center of rotation in electron flowing is a wave source(or a power source). In other words, wave ...
... The essence of gravitational wave is electron, so that light-wave is a kind of gravitational wave full of energy. All the collection of mass can be considered as a set of energy or wave source . And the center of rotation in electron flowing is a wave source(or a power source). In other words, wave ...
SMS-204: Integrative marine sciences.
... Friction is the process through which mechanical energy is converted to heat. The breaks in our car heat when we use them and so do our hands when we rub them against each other. Friction can often be an undesirable conversion of energy to heat. In that process a loss of some energy (to heat, anothe ...
... Friction is the process through which mechanical energy is converted to heat. The breaks in our car heat when we use them and so do our hands when we rub them against each other. Friction can often be an undesirable conversion of energy to heat. In that process a loss of some energy (to heat, anothe ...
physical change
... Each molecule of a compound contains two or more elements that are chemically combined. ...
... Each molecule of a compound contains two or more elements that are chemically combined. ...
CHAPTER 1 -Chemistry -Matter -Elements -Atoms
... III. The enthalpy of the process can be calculated by multiplying the temperature change and heat capacity of the system. IV. The heat that caused the temperature change came from the surroundings. a. I b. II c. I and III d. II and III e. I,III, and IV 1) If a system absorbs 53 kJ of heat and does 3 ...
... III. The enthalpy of the process can be calculated by multiplying the temperature change and heat capacity of the system. IV. The heat that caused the temperature change came from the surroundings. a. I b. II c. I and III d. II and III e. I,III, and IV 1) If a system absorbs 53 kJ of heat and does 3 ...
The First Law of Thermodynamics
... expansion at constant pressure until the volume is 2.5 L, after which is cooled at constant volume until its pressure is 1 atm. It is then compressed at constant pressure until the volume is again 1L, after which it is heated at constant volume until it is back in its original state. Find (a) the wo ...
... expansion at constant pressure until the volume is 2.5 L, after which is cooled at constant volume until its pressure is 1 atm. It is then compressed at constant pressure until the volume is again 1L, after which it is heated at constant volume until it is back in its original state. Find (a) the wo ...
Statistical mechanics
... kinetic theory of gases. In this work, Bernoulli posited the argument, still used to this day, that gases consist of great numbers of molecules moving in all directions, that their impact on a surface causes the gas pressure that we feel, and that what we experience as heat is simply the kinetic ene ...
... kinetic theory of gases. In this work, Bernoulli posited the argument, still used to this day, that gases consist of great numbers of molecules moving in all directions, that their impact on a surface causes the gas pressure that we feel, and that what we experience as heat is simply the kinetic ene ...
FINAL EXAM Review Sheet / Study Guide Honors Chemistry
... 36) How does the energy of an electron change when the electron moves closer to the nucleus? Farther away? ...
... 36) How does the energy of an electron change when the electron moves closer to the nucleus? Farther away? ...
國立台北科技大學 冷凍與空調工程研究所碩士在職專班
... plus the candle) as the system, determine (a) if there is any heat transfer during this burning process and (b) if there is any change in the internal energy of the system. ...
... plus the candle) as the system, determine (a) if there is any heat transfer during this burning process and (b) if there is any change in the internal energy of the system. ...
1.3.5 Spectroscopy Name Symbol Definition SI unit Notes total term
... letters. As for atoms, the spin multiplicity (2S + 1) may be indicated by a left superscript. For linear molecules the value of Ω(= Λ + Σ) may be added as a right subscript (analogous to J for atoms). If the value of Ω is not specified, the term symbols is taken to refer to all component states, and ...
... letters. As for atoms, the spin multiplicity (2S + 1) may be indicated by a left superscript. For linear molecules the value of Ω(= Λ + Σ) may be added as a right subscript (analogous to J for atoms). If the value of Ω is not specified, the term symbols is taken to refer to all component states, and ...
Unit Powerpoint
... the force x the distance over which the motion occurs) or produce heat. Energy comes in many forms- solar, nuclear, and electrical are just a few examples. In chemistry, heat energy is often what we are interested in. ...
... the force x the distance over which the motion occurs) or produce heat. Energy comes in many forms- solar, nuclear, and electrical are just a few examples. In chemistry, heat energy is often what we are interested in. ...
Prescribed Practicals
... Potassium Hydrogen Phthalate (KHP) is an acidic salt often used as the primary standard for the standardization of a base like NaOH. A primary standard like KHP is pure, stable, and has no waters of hydration. It also has a relatively high molar mass that allows for a high accuracy. A solution ...
... Potassium Hydrogen Phthalate (KHP) is an acidic salt often used as the primary standard for the standardization of a base like NaOH. A primary standard like KHP is pure, stable, and has no waters of hydration. It also has a relatively high molar mass that allows for a high accuracy. A solution ...
Balanced Chemical Equation
... • Classify and identify chemical reactions • Write ionic equations for reactions that occur in water ...
... • Classify and identify chemical reactions • Write ionic equations for reactions that occur in water ...
PCSD General Chemistry Pacing Guide
... If Time Permits: Potential Energy Diagrams Equilibrium Reaction Rates ...
... If Time Permits: Potential Energy Diagrams Equilibrium Reaction Rates ...
Comparison of 2008 to 2000 SCH3U_ud
... compounds can be released into the environment, including cadmium, arsenic, sulphur dioxide, and mercury, all of which can endanger the health and safety of local populations. Sample questions: What are some chemical reactions used in the manufacture of paper? How might the reactants or products of ...
... compounds can be released into the environment, including cadmium, arsenic, sulphur dioxide, and mercury, all of which can endanger the health and safety of local populations. Sample questions: What are some chemical reactions used in the manufacture of paper? How might the reactants or products of ...
Chapter 6 Chemical Reactions: An Introduction
... • A chemical change occurs when new substances are made. • Visual clues (permanent): – Color change, precipitate formation, gas bubbles, flames, heat release, cooling, light ...
... • A chemical change occurs when new substances are made. • Visual clues (permanent): – Color change, precipitate formation, gas bubbles, flames, heat release, cooling, light ...
BISMUTH (III) OXIDE - Dudley Chemical Corporation
... no representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the information must exercise their independent judgment in determining its ap ...
... no representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the information must exercise their independent judgment in determining its ap ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.