Reaction Analysis and PAT Tools
... iC IR™ software was designed to take infrared data and convert it into useful and meaningful information about chemical reactions, in real time. The result of an extensive research project on how scientists analyze reactions, iC IR allows chemists and engineers to quickly gain an understanding of th ...
... iC IR™ software was designed to take infrared data and convert it into useful and meaningful information about chemical reactions, in real time. The result of an extensive research project on how scientists analyze reactions, iC IR allows chemists and engineers to quickly gain an understanding of th ...
Covalent Bonding - whitburnscience
... We can replace the words with chemical formula, which can be worked out using the rules of valency and by knowing which elements form diatomic molecules. The equation above becomes Na + Cl2 ...
... We can replace the words with chemical formula, which can be worked out using the rules of valency and by knowing which elements form diatomic molecules. The equation above becomes Na + Cl2 ...
Explaining Cold Fusion
... solid material. These methods involve different materials containing different impurities as well use of different levels of applied energy. A model must show how it can apply to these different conditions and materials by identifying an active feature common to all. This is an extension of Assumpti ...
... solid material. These methods involve different materials containing different impurities as well use of different levels of applied energy. A model must show how it can apply to these different conditions and materials by identifying an active feature common to all. This is an extension of Assumpti ...
CHAPTER 4: PHASE TRANSITIONS
... Superfluids have the unique quality that their atoms are in the same quantum state. This means they all have the same momentum, and if one moves, they all move. This allows superfluids to move without friction through the tiniest of cracks, and superfluid helium will even flow up the sides of a jar ...
... Superfluids have the unique quality that their atoms are in the same quantum state. This means they all have the same momentum, and if one moves, they all move. This allows superfluids to move without friction through the tiniest of cracks, and superfluid helium will even flow up the sides of a jar ...
The Third Law of Quantum Thermodynamics in the Presence of
... a very similar Hamiltonian with coordinate coupling instead. Nevertheless, the thermal noises produced by these couplings have different power spectra, especially at low frequency.[9−11] This provides us a new point of view to understand the specific character of ...
... a very similar Hamiltonian with coordinate coupling instead. Nevertheless, the thermal noises produced by these couplings have different power spectra, especially at low frequency.[9−11] This provides us a new point of view to understand the specific character of ...
104 Homework Packet - Rogue Community College
... Proteins can have extremely high molecular weights, up into the thousands and millions of grams/mole. Calculate the mass of 1 hundred trillion protein molecules if the protein has a molecular weight of 10,000,000 g/mol. Do you think you could see a sample of ...
... Proteins can have extremely high molecular weights, up into the thousands and millions of grams/mole. Calculate the mass of 1 hundred trillion protein molecules if the protein has a molecular weight of 10,000,000 g/mol. Do you think you could see a sample of ...
Isentropic Efficiency in Engineering Thermodynamics Introduction
... gram) rather than molar quantities. The data used are from the NIST program REF PROP (Lemmon et al., 2007), and are slightly different from the data used in Moran and Shapiro. In program REFPROP, the default reference states1 are defined as having zero internal energy and zero entropy so that by def ...
... gram) rather than molar quantities. The data used are from the NIST program REF PROP (Lemmon et al., 2007), and are slightly different from the data used in Moran and Shapiro. In program REFPROP, the default reference states1 are defined as having zero internal energy and zero entropy so that by def ...
Heat
... also involve moles (rather than a mass unit); they are then called molar specific heats. ...
... also involve moles (rather than a mass unit); they are then called molar specific heats. ...
The basic concepts and Thermochemistry
... The internal energy is a state function: its value depends only on the current state of the system and is independent on how this state was prepared. It is a function of properties that determine the physical state of the system, i.e., pressure, volume, temperature, and amount of substance. Changing ...
... The internal energy is a state function: its value depends only on the current state of the system and is independent on how this state was prepared. It is a function of properties that determine the physical state of the system, i.e., pressure, volume, temperature, and amount of substance. Changing ...
q 2 - q 1
... started by increasing the external pressure, which equals PH2O(T), by Δ P, it is possible to show that, for a cycle, the permanent change in the external energy of the heat reservoir is V ΔP. Thus, as ΔP approaches infinitesimal value, i.e. ΔP→ δP, reversibility is approached, i.e. when evaporation ...
... started by increasing the external pressure, which equals PH2O(T), by Δ P, it is possible to show that, for a cycle, the permanent change in the external energy of the heat reservoir is V ΔP. Thus, as ΔP approaches infinitesimal value, i.e. ΔP→ δP, reversibility is approached, i.e. when evaporation ...
Chemistry
... (n) outline the importance of hydrogen bonding to the physical properties of substances, including ice and water (o) suggest from quoted physical data the type of structure and bonding present in a substance (p) recognise that materials are a finite resource and the importance of recycling processes ...
... (n) outline the importance of hydrogen bonding to the physical properties of substances, including ice and water (o) suggest from quoted physical data the type of structure and bonding present in a substance (p) recognise that materials are a finite resource and the importance of recycling processes ...
Starter S-30
... -reactants can be impure -reactions may not go to completion -may compete with smaller “side” reactions In some reactions as little as 60% yield is considered a good result ...
... -reactants can be impure -reactions may not go to completion -may compete with smaller “side” reactions In some reactions as little as 60% yield is considered a good result ...
Stuff Matters Handout
... Matter is everything around you. Matter is anything made of atoms and molecules. Matter is anything that has mass and takes up space. If you are new to the idea of mass, it is the amount of stuff in an object. Matter is sometimes related to light and electromagnetic radiation. Even though matter can ...
... Matter is everything around you. Matter is anything made of atoms and molecules. Matter is anything that has mass and takes up space. If you are new to the idea of mass, it is the amount of stuff in an object. Matter is sometimes related to light and electromagnetic radiation. Even though matter can ...
Chap. 3 Some general statements
... Some general statements • The energy of the universe is constant • The First Law: – defines internal energy – states that heat is a form of energy. – states that energy is conserved ...
... Some general statements • The energy of the universe is constant • The First Law: – defines internal energy – states that heat is a form of energy. – states that energy is conserved ...
The Born-Haber Cycle
... Using the Born-Haber Cycle in this way, we can determine a reasonable estimate for the coulombic attraction between the atoms in almost any ionic ...
... Using the Born-Haber Cycle in this way, we can determine a reasonable estimate for the coulombic attraction between the atoms in almost any ionic ...
do not
... How do enzymes work? 1) Enzymes act upon a substance called a substrate 2) The enzyme has an indent in it called the active site where the substrate can fit into, kind of like a lock and a key ...
... How do enzymes work? 1) Enzymes act upon a substance called a substrate 2) The enzyme has an indent in it called the active site where the substrate can fit into, kind of like a lock and a key ...
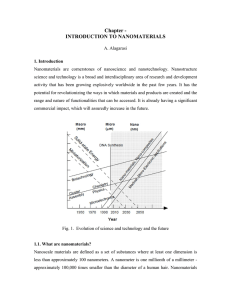
Chapter - INTRODUCTION TO NANOMATERIALS
... unwanted reactant is left on the product and completely removing any growth aids that ...
... unwanted reactant is left on the product and completely removing any growth aids that ...
- Catalyst
... Question 3: Circle the state of matter that has the weakest attractive forces between molecules: Solid Liquid Gas ...
... Question 3: Circle the state of matter that has the weakest attractive forces between molecules: Solid Liquid Gas ...
(General Equilibrium) Part 1
... -The value for the concentrations of solids and liquids are incorporated into the value of Kc. C. Units for Kc – Kc is expressed without units. This is because formally, the value that is put into the Kc expression is not the molar concentration, but the ratio of the concentration to a reference con ...
... -The value for the concentrations of solids and liquids are incorporated into the value of Kc. C. Units for Kc – Kc is expressed without units. This is because formally, the value that is put into the Kc expression is not the molar concentration, but the ratio of the concentration to a reference con ...
Chloropren Anglictina
... Reproductive Hazard (HE5); Systemic Toxicity (HE3),Mutagen (HE2) system, skin, eyes ...
... Reproductive Hazard (HE5); Systemic Toxicity (HE3),Mutagen (HE2) system, skin, eyes ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.