Compounds and Equations
... Chemical Equations use chemical formulas and physical states like solid (s), liquid (l), gases (g), and aqueous (aq) to describe a chemical reaction. These equations give us information about what is reacting, what is being formed and how much or the number of moles of each substance and even the en ...
... Chemical Equations use chemical formulas and physical states like solid (s), liquid (l), gases (g), and aqueous (aq) to describe a chemical reaction. These equations give us information about what is reacting, what is being formed and how much or the number of moles of each substance and even the en ...
Effects of chemical weapons on cancer development in human
... Cancer development is a long process which may take several months to several years to appear. This require victims of the chemical weapon to survive long enough to be monitored for any sign of cancer development. However, people who expose to nerve gases die soon after exposure due to the fact that ...
... Cancer development is a long process which may take several months to several years to appear. This require victims of the chemical weapon to survive long enough to be monitored for any sign of cancer development. However, people who expose to nerve gases die soon after exposure due to the fact that ...
TDB-5: Standards and conventions for TDB publications
... tris(2-pyridylmethyl)amine tetrakis(2-pyridylmethyl)amine 2,2’,2”-triaminotriethylamine triethylenetetraamine tryptophanate thiourea thyrosinate valinate ...
... tris(2-pyridylmethyl)amine tetrakis(2-pyridylmethyl)amine 2,2’,2”-triaminotriethylamine triethylenetetraamine tryptophanate thiourea thyrosinate valinate ...
Questions 1-2
... (A) are made up of atoms that are intrinsically hard because of their electronic structures (B) consist of positive and negative ions that are strongly attracted to each other (C) are giant molecules in which each atom forms strong covalent bonds with all of its neighboring atoms (D) are formed unde ...
... (A) are made up of atoms that are intrinsically hard because of their electronic structures (B) consist of positive and negative ions that are strongly attracted to each other (C) are giant molecules in which each atom forms strong covalent bonds with all of its neighboring atoms (D) are formed unde ...
File
... It’s time to practice what you have already learned about moles, chemical reactions and dimensional analysis. We will learn one new conversion factor and then combine it with other concepts. Molar Volume is the volume of one mole of gas. Since the space between molecules in a gas is very great compa ...
... It’s time to practice what you have already learned about moles, chemical reactions and dimensional analysis. We will learn one new conversion factor and then combine it with other concepts. Molar Volume is the volume of one mole of gas. Since the space between molecules in a gas is very great compa ...
第四章理想气体的热力过程
... The amount of heat added to a closed system during a constant volume process equals to the increase in internal energy. ...
... The amount of heat added to a closed system during a constant volume process equals to the increase in internal energy. ...
Chemistry Test at a Glance
... This assessment includes two tests. You may take either test individually or the full assessment in a single session. The testing time is the amount of time you will have to answer the questions on the test. Test duration includes time for tutorials and directional screens that may be included in th ...
... This assessment includes two tests. You may take either test individually or the full assessment in a single session. The testing time is the amount of time you will have to answer the questions on the test. Test duration includes time for tutorials and directional screens that may be included in th ...
Chapter 1
... 49. Write the empirical formula corresponding to each of the following molecular formulas: a) Al2Br6 AlBr3 b) C8H10 C4H5 c) C4H8O2 C2H4O2 50. Determine the molecular and empirical formulas of the following: a) the organic solvent benzene, which has six carbon atoms and six hydrogen atoms; C6H6, CH b ...
... 49. Write the empirical formula corresponding to each of the following molecular formulas: a) Al2Br6 AlBr3 b) C8H10 C4H5 c) C4H8O2 C2H4O2 50. Determine the molecular and empirical formulas of the following: a) the organic solvent benzene, which has six carbon atoms and six hydrogen atoms; C6H6, CH b ...
Chapter 1 Chemistry and Measurement
... c. Understand the difference between chemical changes (chemical reactions) and physical changes. d. Distinguish between chemical properties and physical properties. Physical Measurements 5. Measurement and Significant Figures a. Define and use the terms precision and accuracy when describing measur ...
... c. Understand the difference between chemical changes (chemical reactions) and physical changes. d. Distinguish between chemical properties and physical properties. Physical Measurements 5. Measurement and Significant Figures a. Define and use the terms precision and accuracy when describing measur ...
Ayres, Bob – Exergy, economic growth and degrowth
... • That investment choices (in the equilibrium model) are always optimal because firms maximize profits and consumers maximize utility, over time. In this case there are no “free lunches” – meaning no investment opportunities that would have negative costs or very low costs compared to typical practi ...
... • That investment choices (in the equilibrium model) are always optimal because firms maximize profits and consumers maximize utility, over time. In this case there are no “free lunches” – meaning no investment opportunities that would have negative costs or very low costs compared to typical practi ...
Enzymes - WordPress.com
... active site is the keyhole of the lock. (3) A process called CATALYSIS happens. Catalysis is when the substrate is changed. It could be broken down or combined with another molecule to make something new. (4) The enzyme lets go. When the enzyme lets go, it returns to normal, ready to do another reac ...
... active site is the keyhole of the lock. (3) A process called CATALYSIS happens. Catalysis is when the substrate is changed. It could be broken down or combined with another molecule to make something new. (4) The enzyme lets go. When the enzyme lets go, it returns to normal, ready to do another reac ...
Chemistry Merit Badge
... A) Visit a laboratory and talk to a practicing chemist. Ask what the chemist does, and what training and education are needed to work as a chemist. B) Using resources found at the library and in periodicals, books, and the Internet (with your parent’s permission), learn about two different kinds of ...
... A) Visit a laboratory and talk to a practicing chemist. Ask what the chemist does, and what training and education are needed to work as a chemist. B) Using resources found at the library and in periodicals, books, and the Internet (with your parent’s permission), learn about two different kinds of ...
Chemistry in Society - Cathkin High School
... distillation. Naphtha is a feedstock that can be cracked to produce ethene. Batch and Continuous Processes In a batch process the chemicals are loaded into the reaction vessel. The reaction is monitored and at the end of the reaction the product is separated and the reaction vessel cleaned out ready ...
... distillation. Naphtha is a feedstock that can be cracked to produce ethene. Batch and Continuous Processes In a batch process the chemicals are loaded into the reaction vessel. The reaction is monitored and at the end of the reaction the product is separated and the reaction vessel cleaned out ready ...
Are You suprised ?
... A system at equilibrium is described by the equation: Heat + SO2Cl2(l ) ⇌ SO2(g) + Cl2(g) One of the following sentences is correct. A) Adding Cl2 will increase heat. B) The equilibrium will move to the left when we remove Cl2. C) Increasing the pressure has no effect on this system. D) Adding catal ...
... A system at equilibrium is described by the equation: Heat + SO2Cl2(l ) ⇌ SO2(g) + Cl2(g) One of the following sentences is correct. A) Adding Cl2 will increase heat. B) The equilibrium will move to the left when we remove Cl2. C) Increasing the pressure has no effect on this system. D) Adding catal ...
Factors that affect the rate of reactions
... liquid, liquid and a gas etc. It does not affect reactants that are in the same phase. To summarize the 3 ways to change the rate of a reaction. If you can make the reactants: MOVE FASTER, HIT each other MORE OFTEN with MORE ENERGY The reaction rate will INCREASE ...
... liquid, liquid and a gas etc. It does not affect reactants that are in the same phase. To summarize the 3 ways to change the rate of a reaction. If you can make the reactants: MOVE FASTER, HIT each other MORE OFTEN with MORE ENERGY The reaction rate will INCREASE ...
KEY Final Exam Review - Iowa State University
... k=(0.2130)M/s/(0.250M)(0.250M)=3.41M-1s-1 could use any of the five to calculate this. kave=3.408M-1s-1 d. What is the rate when [BF3]=0.100M and [NH3]=0.500M? rate=3.408M-1s1*(0.100M)*(0.500M)=0.170M/s 2a. Write the rate law for a reaction between A, B, and C that is the first order in A, zero orde ...
... k=(0.2130)M/s/(0.250M)(0.250M)=3.41M-1s-1 could use any of the five to calculate this. kave=3.408M-1s-1 d. What is the rate when [BF3]=0.100M and [NH3]=0.500M? rate=3.408M-1s1*(0.100M)*(0.500M)=0.170M/s 2a. Write the rate law for a reaction between A, B, and C that is the first order in A, zero orde ...
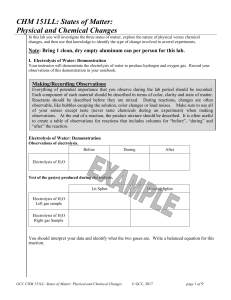
CHM 151LL: States of Matter: Physical and Chemical Changes
... boiling point a liquid will boil and become a gas. If we decrease the temperature, which decreases the molecular motion, a gas will condense into a liquid at the boiling point, and a liquid will freeze to become a solid at the melting point (which can also be called the freezing point). A few substa ...
... boiling point a liquid will boil and become a gas. If we decrease the temperature, which decreases the molecular motion, a gas will condense into a liquid at the boiling point, and a liquid will freeze to become a solid at the melting point (which can also be called the freezing point). A few substa ...
H 2 (g)
... Isolated system is a system that does not allow the exchange of either mass or energy with its surroundings ...
... Isolated system is a system that does not allow the exchange of either mass or energy with its surroundings ...
unit 4 practice
... 2. Equal volumes and concentrations of hydrochloric acid and ethanoic acid are titrated with sodium hydroxide solutions of the same concentration. Which statement is correct? A. The initial pH values of ...
... 2. Equal volumes and concentrations of hydrochloric acid and ethanoic acid are titrated with sodium hydroxide solutions of the same concentration. Which statement is correct? A. The initial pH values of ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.