The Second Law of Thermodynamics
... floor. To describe this process in another way, we say that the original potential energy of the ball, through its conversion to kinetic energy, is degraded into heat. Now let us consider what would be necessary for the reverse process to occur on its own; that is, a ball sitting on the floor spontane ...
... floor. To describe this process in another way, we say that the original potential energy of the ball, through its conversion to kinetic energy, is degraded into heat. Now let us consider what would be necessary for the reverse process to occur on its own; that is, a ball sitting on the floor spontane ...
Document
... Consider the following balanced reaction (NH4)2S(s) 2NH3(g) + H2S(g) An equilibrium mixture of this mixture at a certain temperature was found to have [NH3] = 0.278 M and [H2S] = 0.355 M. What is the value of the equilibrium constant (Kc) at this temperature? ...
... Consider the following balanced reaction (NH4)2S(s) 2NH3(g) + H2S(g) An equilibrium mixture of this mixture at a certain temperature was found to have [NH3] = 0.278 M and [H2S] = 0.355 M. What is the value of the equilibrium constant (Kc) at this temperature? ...
aq - HCC Learning Web
... • Aqueous solutions of lead(II) nitrate and potassium iodide produce a yellow precipitate of lead(II) iodide and an aqueous solution of potassium nitrate Pb(NO3)2(aq) + KI(aq) PbI2(s) + KNO3(aq) • Aqueous solutions of calcium nitrate and sodium carbonate react to give a white precipitate of calciu ...
... • Aqueous solutions of lead(II) nitrate and potassium iodide produce a yellow precipitate of lead(II) iodide and an aqueous solution of potassium nitrate Pb(NO3)2(aq) + KI(aq) PbI2(s) + KNO3(aq) • Aqueous solutions of calcium nitrate and sodium carbonate react to give a white precipitate of calciu ...
Stoichiometry Regents Unit Review
... 6. Given the reaction: PbCl2(aq) + Na2CrO4(aq) → PbCrO4(S) + 2 NaCl(aq) What is the total number of moles of NaCl formed when 2 moles of Na2CrO4 react completely? (1) 1mole (2) 2 moles (3) 3 moles (4) 4 moles 7 What is conserved during a chemical reaction? (1) mass, only (2) charge, only (3) both ma ...
... 6. Given the reaction: PbCl2(aq) + Na2CrO4(aq) → PbCrO4(S) + 2 NaCl(aq) What is the total number of moles of NaCl formed when 2 moles of Na2CrO4 react completely? (1) 1mole (2) 2 moles (3) 3 moles (4) 4 moles 7 What is conserved during a chemical reaction? (1) mass, only (2) charge, only (3) both ma ...
lecture slides of chap19_FU
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Unit 9 Chemical Equations and Reactions Balancing Equations Notes
... of the process that occurs in a chemical reaction. A chemical equation is written with the _______________ (starting materials) on the left side of an arrow and the ___________________ (resulting substance) of the chemical reaction on the right side of the equation. The head of the arrow typically p ...
... of the process that occurs in a chemical reaction. A chemical equation is written with the _______________ (starting materials) on the left side of an arrow and the ___________________ (resulting substance) of the chemical reaction on the right side of the equation. The head of the arrow typically p ...
Lecture 14 Chapter 19 Ideal Gas Law and Kinetic Theory of Gases
... Note that units are Joule per kelvin and the sign is the same as Q since T > 0 • However, the above formula can only be used to calculate the entropy change if the process is reversible.. • To find the entropy for an irreversible process and since state functions only depend on the end points, the t ...
... Note that units are Joule per kelvin and the sign is the same as Q since T > 0 • However, the above formula can only be used to calculate the entropy change if the process is reversible.. • To find the entropy for an irreversible process and since state functions only depend on the end points, the t ...
7.1 Describing Reactions
... If you examine this equation carefully, you will notice that the number of atoms on the left side does not equal the number of atoms on the right. The equation is not balanced. In order to show that mass is conserved during a reaction, a chemical equation must be balanced. You can balance a chemical ...
... If you examine this equation carefully, you will notice that the number of atoms on the left side does not equal the number of atoms on the right. The equation is not balanced. In order to show that mass is conserved during a reaction, a chemical equation must be balanced. You can balance a chemical ...
molecular vibrations: from harmonic oscillators to pendulums
... transition state theory of reaction rates, formulated by Wigner, Eyring, and others in the 1930s, is actually based on classical mechanics. At the same time it is a sobering thought that quantum mechanics, the correct theory for microscopic systems, is less than a century old but organic and inorgan ...
... transition state theory of reaction rates, formulated by Wigner, Eyring, and others in the 1930s, is actually based on classical mechanics. At the same time it is a sobering thought that quantum mechanics, the correct theory for microscopic systems, is less than a century old but organic and inorgan ...
Syllabus_summer 2014_1411_ZF_learning web
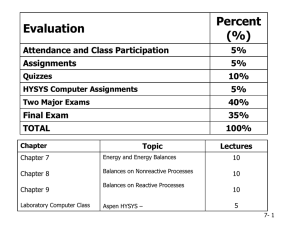
... Quizzes will be given at the very beginning of class (to encourage punctuality) and are designed to check that the students are keeping up with the textbook reading and are able to utilize the material in the textbook (text, tables, figures, sample problems). Missed quizzes can not be made up. The t ...
... Quizzes will be given at the very beginning of class (to encourage punctuality) and are designed to check that the students are keeping up with the textbook reading and are able to utilize the material in the textbook (text, tables, figures, sample problems). Missed quizzes can not be made up. The t ...
Maths for Chemistry Facts and Formulae
... state variables, F , chosen from amongst temperature, pressure and species compositions in each phase, which must be specified to fix the thermodynamic state of a system in equilibrium. Clapeyron equation relates change in pressure to change in temperature at a phase boundary. The slope of the phase ...
... state variables, F , chosen from amongst temperature, pressure and species compositions in each phase, which must be specified to fix the thermodynamic state of a system in equilibrium. Clapeyron equation relates change in pressure to change in temperature at a phase boundary. The slope of the phase ...
Stoichiometry - hrsbstaff.ednet.ns.ca
... writing the general formulas for alkanes, alkenes (one double bond), alkadienes (two double bonds), alkynes (one triple bond), nonsubstituted cycloalkanes and cycloalkenes defining and being able to give examples of saturated and unsaturated hydrocarbons being able to name all the prefixes for ...
... writing the general formulas for alkanes, alkenes (one double bond), alkadienes (two double bonds), alkynes (one triple bond), nonsubstituted cycloalkanes and cycloalkenes defining and being able to give examples of saturated and unsaturated hydrocarbons being able to name all the prefixes for ...
Material Safety Data Sheet - Dudley Chemical Corporation
... DUDLEY CORPORATION provides the information herein in good faith but makes no representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the ...
... DUDLEY CORPORATION provides the information herein in good faith but makes no representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the ...
Problem Set: Empirical and Molecular Formulas
... 6. Titanium (IV) oxide, TiO2, is used as a pigment in paints and as a whitening and coating agent for paper. It can be made by reacting O2 with TiCl4. TiCl4 + O2 TiO2 + 2 Cl2 (already balanced) a) If 4.5 mol of TiCl4 react with 3.5 mol O2, identify both the limiting and excess reactants. b) How ma ...
... 6. Titanium (IV) oxide, TiO2, is used as a pigment in paints and as a whitening and coating agent for paper. It can be made by reacting O2 with TiCl4. TiCl4 + O2 TiO2 + 2 Cl2 (already balanced) a) If 4.5 mol of TiCl4 react with 3.5 mol O2, identify both the limiting and excess reactants. b) How ma ...
CHEM-4511-01
... There may be some voluntary additional lectures to cover some material in more depth, review some math and physics, or do some computer experiments to enhance learning. In that case, time and place is to be announced. ...
... There may be some voluntary additional lectures to cover some material in more depth, review some math and physics, or do some computer experiments to enhance learning. In that case, time and place is to be announced. ...
Revised Syllabus - M. Sc. First Year - Chemistry
... b) Thermodynamics of the reaction, kinetic of the reaction, thermodynamic verses kinetic control of reactions, Hammett and Taft effect. c) Kinetic isotopic effects, method of determining reaction mechanism d) Structure and stability of reactive intermediates, carbenes, nitrenes, carbocations, carban ...
... b) Thermodynamics of the reaction, kinetic of the reaction, thermodynamic verses kinetic control of reactions, Hammett and Taft effect. c) Kinetic isotopic effects, method of determining reaction mechanism d) Structure and stability of reactive intermediates, carbenes, nitrenes, carbocations, carban ...
Chemical Formulas and Chemical Compounds
... In general, if the anion name ends in -ate, the corresponding acid name will end in a suffix of -ic. In general, if the anion name ends in -ite, the corresponding acid name will end in a suffix of -ous. b. Derive a generalization for determining whether an acid name will begin with the prefix hydroo ...
... In general, if the anion name ends in -ate, the corresponding acid name will end in a suffix of -ic. In general, if the anion name ends in -ite, the corresponding acid name will end in a suffix of -ous. b. Derive a generalization for determining whether an acid name will begin with the prefix hydroo ...
Which notation represents an atom of sodium
... a) The equation represents a physical change, with the product and reactants having different chemical properties. b) The equation represents a physical change, with the product and reactants having identical chemical properties. c) The equation represents a chemical change, with the product and rea ...
... a) The equation represents a physical change, with the product and reactants having different chemical properties. b) The equation represents a physical change, with the product and reactants having identical chemical properties. c) The equation represents a chemical change, with the product and rea ...
Calorimetry
... Rules for manipulating thermochemical equations: 1. When an equation is reversed, the Hof must also be reversed. 2. Formulas cancelled from both sides of the equation must be the same substance in the same physical state. 3. If all the coefficients of an equation are multiplied or divided by the sam ...
... Rules for manipulating thermochemical equations: 1. When an equation is reversed, the Hof must also be reversed. 2. Formulas cancelled from both sides of the equation must be the same substance in the same physical state. 3. If all the coefficients of an equation are multiplied or divided by the sam ...
Slide 1
... In a similar way, a calcium atom may lose two electrons to two chlorine atoms forming a calcium ion Ca2+ and two chloride ions Cl-, that is calcium chloride CaCl2 : ...
... In a similar way, a calcium atom may lose two electrons to two chlorine atoms forming a calcium ion Ca2+ and two chloride ions Cl-, that is calcium chloride CaCl2 : ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.