Thermodynamics of Equilibrium
... value of the equilibrium constant of a reaction solely from information about the products and reactants themselves, without any knowledge at all about the mechanism or other details of the reaction? The answer is yes, and this turns out to be the central purpose of chemical thermodynamics: The purp ...
... value of the equilibrium constant of a reaction solely from information about the products and reactants themselves, without any knowledge at all about the mechanism or other details of the reaction? The answer is yes, and this turns out to be the central purpose of chemical thermodynamics: The purp ...
Exam 1
... The best description of the effect of a catalyst on a chemical reaction is that it A. lowers the activation energy of the forward reaction without changing the activation energy of the reverse reaction. B. lowers the activation energy of the forward reaction and raises the activation energy of the r ...
... The best description of the effect of a catalyst on a chemical reaction is that it A. lowers the activation energy of the forward reaction without changing the activation energy of the reverse reaction. B. lowers the activation energy of the forward reaction and raises the activation energy of the r ...
Final Study Guide (Semester 2) Answer Key
... ***The first thing you should do when solving this is look at the common ion chart and write down all the ions. It’s much easier than looking them up again for each question. a. Write the balanced molecular equation. Include phase symbols. Ba(NO3)2(aq) + K2SO4(aq) BaSO4(s) + 2KNO3 (aq) Switch the c ...
... ***The first thing you should do when solving this is look at the common ion chart and write down all the ions. It’s much easier than looking them up again for each question. a. Write the balanced molecular equation. Include phase symbols. Ba(NO3)2(aq) + K2SO4(aq) BaSO4(s) + 2KNO3 (aq) Switch the c ...
Honors Chemistry / SAT II
... 2488. The maximum numbers of electrons in the K, L, M, and N shells of any element are respectively (A) 1, 2, 8, 16 (D) 2, 8, 18, 32 (B) 1, 4, 9, 16 (E) 2, 6, 10, 14 (C) 2, 8, 16, 24 ...
... 2488. The maximum numbers of electrons in the K, L, M, and N shells of any element are respectively (A) 1, 2, 8, 16 (D) 2, 8, 18, 32 (B) 1, 4, 9, 16 (E) 2, 6, 10, 14 (C) 2, 8, 16, 24 ...
Document
... Another way to state Hess’s law is: If two or more equations with known enthalpy changes can be added together to form a new “target” equation, then their enthalpy changes may be similarly added together to yield the enthalpy change of the target equation. Hess’s law can also be written as an equati ...
... Another way to state Hess’s law is: If two or more equations with known enthalpy changes can be added together to form a new “target” equation, then their enthalpy changes may be similarly added together to yield the enthalpy change of the target equation. Hess’s law can also be written as an equati ...
File
... - in phase equilibrium particles in both phases are gaining or losing kinetic energy, such that they are ________ from one phase to another, while an equal number are moving in the reverse direction e.g. H2O(l) → H20(g) 3. Chemical Reaction Equilibrium - Quantitative reactions are those reactions wh ...
... - in phase equilibrium particles in both phases are gaining or losing kinetic energy, such that they are ________ from one phase to another, while an equal number are moving in the reverse direction e.g. H2O(l) → H20(g) 3. Chemical Reaction Equilibrium - Quantitative reactions are those reactions wh ...
Basic Physical Chemistry (12.4 MB ppt)
... It enables to determine the caloric value of foods by their burning up, although in human body are they metabolized in plenty of gradual steps (glucose, lipids etc.). Calculation of reaction heat is realized from combination heats (heat, which is released (consumed) by formation o 1 mol of the compo ...
... It enables to determine the caloric value of foods by their burning up, although in human body are they metabolized in plenty of gradual steps (glucose, lipids etc.). Calculation of reaction heat is realized from combination heats (heat, which is released (consumed) by formation o 1 mol of the compo ...
Name: 1) What is the oxidation number of sulfur in H SO ? A)
... In any oxidation-reduction reaction, the total number of electrons gained is A) greater than the total number of electrons lost B) equal to the total number of electrons lost ...
... In any oxidation-reduction reaction, the total number of electrons gained is A) greater than the total number of electrons lost B) equal to the total number of electrons lost ...
Enzymes: “Helper” Protein molecules
... Enzymes aren’t used up Enzymes are not changed by the reaction used only temporarily re-used again for the same reaction with other molecules very little enzyme needed to help in many reactions ...
... Enzymes aren’t used up Enzymes are not changed by the reaction used only temporarily re-used again for the same reaction with other molecules very little enzyme needed to help in many reactions ...
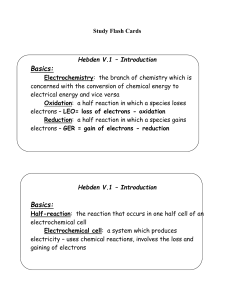
Hebden V.2 – Oxidation Numbers
... Reactions are spontaneous only if 1. the reactant to be reduced (the oxidizing agent) is above the reactant to be oxidized (the reducing agent). 2. both reactants are on the same side = no reaction 3. the reducing agent is above the oxidizing agent = no reaction Check for H+ - for many reactions – H ...
... Reactions are spontaneous only if 1. the reactant to be reduced (the oxidizing agent) is above the reactant to be oxidized (the reducing agent). 2. both reactants are on the same side = no reaction 3. the reducing agent is above the oxidizing agent = no reaction Check for H+ - for many reactions – H ...
CB-80 - Rove Pest Control
... WARRANTY, EXPRESSED OR IMPLIED, IS MADE CONCERNING THE INFORMATION PROVIDED HEREIN. The information provided herein relates only to the specified product designated and may not be applicable where such product is used in combination with any other materials or in any process. , Use of this product i ...
... WARRANTY, EXPRESSED OR IMPLIED, IS MADE CONCERNING THE INFORMATION PROVIDED HEREIN. The information provided herein relates only to the specified product designated and may not be applicable where such product is used in combination with any other materials or in any process. , Use of this product i ...
Document
... between objects, or converted from one form to another, the total amount of energy present at the beginning must be present at the end. © 2014 Pearson Education, Inc. ...
... between objects, or converted from one form to another, the total amount of energy present at the beginning must be present at the end. © 2014 Pearson Education, Inc. ...
Chemical Stoichiometry
... • Equations should be balanced • Have the same number of each kind of atoms on both sides because ... ...
... • Equations should be balanced • Have the same number of each kind of atoms on both sides because ... ...
Chapter 5
... reactions, energy, often in the form of heat, is absorbed—that is, it acts somewhat like a reactant. You might write an equation for those reactions that looks like this: energy + reactants S products In other reactions, energy is produced—that is, it acts like a product: reactants S products + ener ...
... reactions, energy, often in the form of heat, is absorbed—that is, it acts somewhat like a reactant. You might write an equation for those reactions that looks like this: energy + reactants S products In other reactions, energy is produced—that is, it acts like a product: reactants S products + ener ...
Chemistry Senior External Syllabus 1998
... Candidates will be assessed by means of two examinations, and details of the papers are provided in section 8. While direct assessment of the suggested experiments and manipulative skills stated in the syllabus is not possible, they will be assessed indirectly in the examination papers. Consequently ...
... Candidates will be assessed by means of two examinations, and details of the papers are provided in section 8. While direct assessment of the suggested experiments and manipulative skills stated in the syllabus is not possible, they will be assessed indirectly in the examination papers. Consequently ...
3. What is the empirical formula of a compound that is
... of energy from molecules containing little or no oxygen prior to oxidation? Wouldn't we guess that a molecule such as glucose, already containing 53% oxygen, would produce less energy than fats that might contain 11% oxygen? (Modified from General Chemistry Case Studies) Consider the oxidation of a ...
... of energy from molecules containing little or no oxygen prior to oxidation? Wouldn't we guess that a molecule such as glucose, already containing 53% oxygen, would produce less energy than fats that might contain 11% oxygen? (Modified from General Chemistry Case Studies) Consider the oxidation of a ...
Atomic Theory
... Isotopes ..................................................................................................................................................................................................4 Calculating RAM by example – Lead (Pb)......................................................... ...
... Isotopes ..................................................................................................................................................................................................4 Calculating RAM by example – Lead (Pb)......................................................... ...
Table of Contents - Free Coursework for GCSE, IGCSE, A Level, IB
... Isotopes ..................................................................................................................................................................................................4 Calculating RAM by example – Lead (Pb)......................................................... ...
... Isotopes ..................................................................................................................................................................................................4 Calculating RAM by example – Lead (Pb)......................................................... ...
College Chemistry I PHS 1025 Fall 2012 Practice Exam 3A
... containing 500. g of water, the temperature of the water increases by 8.63°C. Assuming that the specific heat of water is 4.18 J/(g ∙ °C), and that the heat absorption by the calorimeter is negligible, estimate the enthalpy of combustion per mole of anthracene. A) -7070 kJ/mol B) +39.7 kJ/mol C) -81 ...
... containing 500. g of water, the temperature of the water increases by 8.63°C. Assuming that the specific heat of water is 4.18 J/(g ∙ °C), and that the heat absorption by the calorimeter is negligible, estimate the enthalpy of combustion per mole of anthracene. A) -7070 kJ/mol B) +39.7 kJ/mol C) -81 ...
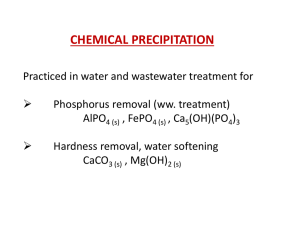
Phosphorus Removal from Wastewater by Chemical Precipitation
... The chemistry of phosphate precipitate formation is complex because of complexes formed between phosphate and metals, and between metals and other ligands in the wastewater. Side reactions of the metals with alkalinity to form hydroxide precipitates are another factor to be considered. The am ...
... The chemistry of phosphate precipitate formation is complex because of complexes formed between phosphate and metals, and between metals and other ligands in the wastewater. Side reactions of the metals with alkalinity to form hydroxide precipitates are another factor to be considered. The am ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.