1. What is the best definition of rate of reaction? A. The time it takes
... Nitrogen(II) oxide reacts with hydrogen according to the following equation: 2NO(g) + 2H2(g) → N2(g) + 2H2O(g) The table shows how the rate of reaction varies as the concentrations of the reactants are ...
... Nitrogen(II) oxide reacts with hydrogen according to the following equation: 2NO(g) + 2H2(g) → N2(g) + 2H2O(g) The table shows how the rate of reaction varies as the concentrations of the reactants are ...
Table of Contents Pages Unit 1- Matter and Change 1
... Homogeneous mixtures are called ___________________. A heterogeneous mixture contains regions that have ____________________ properties from those of other regions. When we pour sand into water, the resulting mixture contains two distinct regions. ___________________ pavement, which has small rocks ...
... Homogeneous mixtures are called ___________________. A heterogeneous mixture contains regions that have ____________________ properties from those of other regions. When we pour sand into water, the resulting mixture contains two distinct regions. ___________________ pavement, which has small rocks ...
File
... • A chemical equation is a way of representing a chemical change. • It shows reactants and products. • To balance an equation means to change the numbers of each molecule involved, so that the same number of atoms of each element appear on the reactants side and on the products side. • Chemical equa ...
... • A chemical equation is a way of representing a chemical change. • It shows reactants and products. • To balance an equation means to change the numbers of each molecule involved, so that the same number of atoms of each element appear on the reactants side and on the products side. • Chemical equa ...
The impact of structural Fe(III) reduction by bacteria on
... trace metals (Ponnamperuma et al., 1967; Roden and Edmonds, 1997), and in the fate of organic contaminants (Lovley et al., 1994; Anderson and Lovley, 1997) in soils and sediments. In freshwater environments such as soils, Fe reduction is likely to be an important terminal electron accepting process ...
... trace metals (Ponnamperuma et al., 1967; Roden and Edmonds, 1997), and in the fate of organic contaminants (Lovley et al., 1994; Anderson and Lovley, 1997) in soils and sediments. In freshwater environments such as soils, Fe reduction is likely to be an important terminal electron accepting process ...
TEKS 8 - UNT College of Education
... molecules, or rearrangement of atoms within molecules. In order to make transformations possible, chemical reactions usually involve the making or breaking of chemical bonds. This does not mean that new elements have been made. In order to make new elements, the nuclear contents must change with the ...
... molecules, or rearrangement of atoms within molecules. In order to make transformations possible, chemical reactions usually involve the making or breaking of chemical bonds. This does not mean that new elements have been made. In order to make new elements, the nuclear contents must change with the ...
1.9 M - Thierry Karsenti
... 2. Atom: the smallest particle of an element that retains the identify and properties of the element and can take part in a chemical change. 3. Atomic number (symbol Z): the number of protons in the nucleus of each atom. 4. Compound: a substance that is formed when two or more elements combine chemi ...
... 2. Atom: the smallest particle of an element that retains the identify and properties of the element and can take part in a chemical change. 3. Atomic number (symbol Z): the number of protons in the nucleus of each atom. 4. Compound: a substance that is formed when two or more elements combine chemi ...
Chapter 2: Mass Relations in Formulas, Chemical Reactions, and
... Section 2.19.4: Balancing the Chemical Reaction: CO2(g) + H2O(l) C6H12O6(s) + O2(g) To balance a chemical reaction, we always start balancing the elements that are present in the least number of compounds. In the above equation, we can start with either carbon or hydrogen. Balancing the element c ...
... Section 2.19.4: Balancing the Chemical Reaction: CO2(g) + H2O(l) C6H12O6(s) + O2(g) To balance a chemical reaction, we always start balancing the elements that are present in the least number of compounds. In the above equation, we can start with either carbon or hydrogen. Balancing the element c ...
1.8 Thermodynamics
... The entropy contribution depends on temperature, T (K) at which the reaction takes place. TDS ...
... The entropy contribution depends on temperature, T (K) at which the reaction takes place. TDS ...
Table of Contents
... which the composition is _______________________, there are no chunks or layers. Salt water, ___________________ ___________________ and dust free air (mixture of nitrogen, oxygen, argon, carbon dioxide, water vapor and other gases) are examples of homogeneous mixtures. Brass (solid mixture of coppe ...
... which the composition is _______________________, there are no chunks or layers. Salt water, ___________________ ___________________ and dust free air (mixture of nitrogen, oxygen, argon, carbon dioxide, water vapor and other gases) are examples of homogeneous mixtures. Brass (solid mixture of coppe ...
Module 2 Alcohols, halogenoalkanes and analysis
... Fermentation is another method for manufacturing alcohol. Carbohydrates are converted into ethanol and carbon dioxide. Sugar or starch is usually used as the carbohydrate source. Ethanol solution produced in this way has a concentration of up to 14% alcohol by volume. Fermentation is carried out in ...
... Fermentation is another method for manufacturing alcohol. Carbohydrates are converted into ethanol and carbon dioxide. Sugar or starch is usually used as the carbohydrate source. Ethanol solution produced in this way has a concentration of up to 14% alcohol by volume. Fermentation is carried out in ...
Topic 14 – Fertilisers – Learning Outcomes
... plants. When living things excrete waste and die, bacteria decompose the material putting nitrogen into the soil and the air. Fertilisers and bacteria in nodules can put nitrogen back into the soil for plants and living things to eat. Nitrogen is lost from this cycle by human waste treatment where t ...
... plants. When living things excrete waste and die, bacteria decompose the material putting nitrogen into the soil and the air. Fertilisers and bacteria in nodules can put nitrogen back into the soil for plants and living things to eat. Nitrogen is lost from this cycle by human waste treatment where t ...
Slide 1
... Its reactions with halide ions in aqueous solution during the formation of the Hydrogen Halides. [CARE: CARRY OUT ALL OF THESE IN A FUME CUPBOARD WITH GREAT CARE] ...
... Its reactions with halide ions in aqueous solution during the formation of the Hydrogen Halides. [CARE: CARRY OUT ALL OF THESE IN A FUME CUPBOARD WITH GREAT CARE] ...
GCE Getting Started - Edexcel
... Reinforcing knowledge, skills and literacy in chemistry From our research, we know that it is easy for teachers to fall into the trap of going over work that has already been covered extensively at KS4. This may be because of a feeling that during the summer break students have forgotten what they h ...
... Reinforcing knowledge, skills and literacy in chemistry From our research, we know that it is easy for teachers to fall into the trap of going over work that has already been covered extensively at KS4. This may be because of a feeling that during the summer break students have forgotten what they h ...
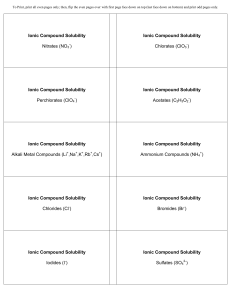
Ionic Compound Solubility Nitrates (NO3 ) Ionic Compound
... Patterns of Chemical Reactivity Definition A substance that is able to donate a H+ ion (a proton) and, hence, increases the concentration of H+(aq) when it dissolves in water. ...
... Patterns of Chemical Reactivity Definition A substance that is able to donate a H+ ion (a proton) and, hence, increases the concentration of H+(aq) when it dissolves in water. ...
1 Introduction
... Nearly a whole kilogram of waste for every kilogram of product! Remember, this is for the ideal case of 100% yield and 100% selectivity. In real life, the E-factor is usually much higher, because product yields are less than 100% and the reagents are often used in excess. Furthermore, in many cases ...
... Nearly a whole kilogram of waste for every kilogram of product! Remember, this is for the ideal case of 100% yield and 100% selectivity. In real life, the E-factor is usually much higher, because product yields are less than 100% and the reagents are often used in excess. Furthermore, in many cases ...
Kinetic investigation of low-pH Fe(II) oxidation and development of a
... organic matter and simultaneous production of energy through biogas production. Biogas is a sub-product of anaerobic digestion that has a high energy value attributed to its high methane content. The use of this gas as a fuel can decrease both energy costs and operational costs in waste treatment pl ...
... organic matter and simultaneous production of energy through biogas production. Biogas is a sub-product of anaerobic digestion that has a high energy value attributed to its high methane content. The use of this gas as a fuel can decrease both energy costs and operational costs in waste treatment pl ...
1aUnit Two Handouts - Dunmore High School
... CaSO4 Fe(C2H3O2)2 K2SO4 HNO3 H2CO3 ZnS NOTE: When H2CO3, H2SO3 or NH4OH are formed as products, they do break down, though not into ions. They break down into H2O and a gaseous substance. ...
... CaSO4 Fe(C2H3O2)2 K2SO4 HNO3 H2CO3 ZnS NOTE: When H2CO3, H2SO3 or NH4OH are formed as products, they do break down, though not into ions. They break down into H2O and a gaseous substance. ...
Chapter12
... b. number of molecules - the balanced equation shows that 1 molecule of nitrogen reacts with 3 molecules of hydrogen in order to form 2 molecules of ammonia. The ratio of molecules of N2:H2:NH3 is always 1:3:2. This means that if you could get 10 molecules of nitrogen to react with 30 molecules of h ...
... b. number of molecules - the balanced equation shows that 1 molecule of nitrogen reacts with 3 molecules of hydrogen in order to form 2 molecules of ammonia. The ratio of molecules of N2:H2:NH3 is always 1:3:2. This means that if you could get 10 molecules of nitrogen to react with 30 molecules of h ...
support material
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and other properties ...
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and other properties ...
Role of Chemical Reaction Engineering in Sustainable
... often combined with novel reactor technology for the process to be economically viable and environmentally beneficial. Hence catalyst development and reactor choice often have to be considered in unison. As a general heuristic rule, the processes that replace liquid routes by solid catalyzed routes ...
... often combined with novel reactor technology for the process to be economically viable and environmentally beneficial. Hence catalyst development and reactor choice often have to be considered in unison. As a general heuristic rule, the processes that replace liquid routes by solid catalyzed routes ...
Review - gbschemphys
... Which of the following statements best describes what a limiting reactant is for any given reaction? a. It is the reactant that is used up first while other reactants remain. b. It is the reactant that has the highest coefficient in the balanced chemical equation. c. It is the reactant that has the ...
... Which of the following statements best describes what a limiting reactant is for any given reaction? a. It is the reactant that is used up first while other reactants remain. b. It is the reactant that has the highest coefficient in the balanced chemical equation. c. It is the reactant that has the ...
Precision, accuracy and significant figures
... For a quantity to have an exact value, it must either be defined or obtained by counting. All measured quantities have an inherent uncertainty because all instruments used to make measurements have limitations, and the people operating the instruments have varying skills. The accuracy of a measureme ...
... For a quantity to have an exact value, it must either be defined or obtained by counting. All measured quantities have an inherent uncertainty because all instruments used to make measurements have limitations, and the people operating the instruments have varying skills. The accuracy of a measureme ...
STOICHIOMETRY:
... The word stoichiometry derives from two Greek words: stoicheion (meaning "element") and metron (meaning "measure"). Stoichiometry deals with calculations about the masses, volumes or concentrations of reactants and products involved in a chemical reaction. The reason we balance chemical reactions is ...
... The word stoichiometry derives from two Greek words: stoicheion (meaning "element") and metron (meaning "measure"). Stoichiometry deals with calculations about the masses, volumes or concentrations of reactants and products involved in a chemical reaction. The reason we balance chemical reactions is ...
TDB-5: Standards and conventions for TDB publications
... • The designator (aq) is used for undissociated, uncharged aqueous species, e.g., U(OH)4 (aq), CO2 (aq). Since ionic gases are not considered in this review, all ions may be assumed to be aqueous and are not designed with (aq). If a chemical reaction refers to a medium other than H2 O (e.g., D2 O, 9 ...
... • The designator (aq) is used for undissociated, uncharged aqueous species, e.g., U(OH)4 (aq), CO2 (aq). Since ionic gases are not considered in this review, all ions may be assumed to be aqueous and are not designed with (aq). If a chemical reaction refers to a medium other than H2 O (e.g., D2 O, 9 ...
Redox

Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between species. The term ""redox"" comes from two concepts involved with electron transfer: reduction and oxidation. It can be explained in simple terms: Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.Although oxidation reactions are commonly associated with the formation of oxides from oxygen molecules, these are only specific examples of a more general concept of reactions involving electron transfer.Redox reactions, or oxidation-reduction reactions, have a number of similarities to acid–base reactions. Like acid–base reactions, redox reactions are a matched set, that is, there cannot be an oxidation reaction without a reduction reaction happening simultaneously. The oxidation alone and the reduction alone are each called a half-reaction, because two half-reactions always occur together to form a whole reaction. When writing half-reactions, the gained or lost electrons are typically included explicitly in order that the half-reaction be balanced with respect to electric charge.Though sufficient for many purposes, these descriptions are not precisely correct. Oxidation and reduction properly refer to a change in oxidation state — the actual transfer of electrons may never occur. The oxidation state of an atom is the fictitious charge that an atom would have if all bonds between atoms of different elements were 100% ionic. Thus, oxidation is better defined as an increase in oxidation state, and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always cause a change in oxidation state, but there are many reactions that are classed as ""redox"" even though no electron transfer occurs (such as those involving covalent bonds).There are simple redox processes, such as the oxidation of carbon to yield carbon dioxide (CO2) or the reduction of carbon by hydrogen to yield methane (CH4), and more complex processes such as the oxidation of glucose (C6H12O6) in the human body through a series of complex electron transfer processes.