Minerals
... a mixture in which one substance dissolves in another. When a hot water solution begins to cool, the elements and compounds leave the solution and begin to crystallize as minerals. Pure metals that crystallize underground form veins. A vein is a narrow channel or slab of a mineral that is different ...
... a mixture in which one substance dissolves in another. When a hot water solution begins to cool, the elements and compounds leave the solution and begin to crystallize as minerals. Pure metals that crystallize underground form veins. A vein is a narrow channel or slab of a mineral that is different ...
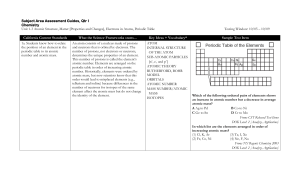
Subject Area Assessment Guides
... electronegativities can form covalent bonds to electrons to form ionic bonds. become molecules. In a covalent bond, therefore, bonding electron pairs are localized in the region between the bonded atoms. In metals valence electrons are not localized to individual atoms but are free to move to tempor ...
... electronegativities can form covalent bonds to electrons to form ionic bonds. become molecules. In a covalent bond, therefore, bonding electron pairs are localized in the region between the bonded atoms. In metals valence electrons are not localized to individual atoms but are free to move to tempor ...
Chemistry – V – BSC – 503
... 1. To determine normality of xN HCl by pH metry. 2. To determine normality and dissociation constant of weak acid (xN CH3COOH) by pH metry. 3. To determine normality and dissociation constant of dibasic acid (xN oxalic acid/malonic acid/maleic acid) using 0.1N NaOH Solution. Colourimetry 1. Find out ...
... 1. To determine normality of xN HCl by pH metry. 2. To determine normality and dissociation constant of weak acid (xN CH3COOH) by pH metry. 3. To determine normality and dissociation constant of dibasic acid (xN oxalic acid/malonic acid/maleic acid) using 0.1N NaOH Solution. Colourimetry 1. Find out ...
5H2O → CuSO4 + 5H2O(g)
... Most reactions in general chemistry take place in an aqueous environment. What does that mean? Terms: ◦ Solution: homogeneous mixture of two or more substances ◦ Solute: substance present in smaller amount ◦ Solvent: substance present in greater amount ◦ Aqueous solution: solvent is water ...
... Most reactions in general chemistry take place in an aqueous environment. What does that mean? Terms: ◦ Solution: homogeneous mixture of two or more substances ◦ Solute: substance present in smaller amount ◦ Solvent: substance present in greater amount ◦ Aqueous solution: solvent is water ...
equilibrium questions - Southington Public Schools
... (ii) Write the solubility-product constant expression for M(OH)2. (iii) Calculate the value of the solubility-product constant, Ksp for M(OH)2 at 25°C. (c) For the metal carbonate, MCO3, the value of the solubility-product constant, Ksp is 7.4×10-14 at 25°C. On the basis of this information and your ...
... (ii) Write the solubility-product constant expression for M(OH)2. (iii) Calculate the value of the solubility-product constant, Ksp for M(OH)2 at 25°C. (c) For the metal carbonate, MCO3, the value of the solubility-product constant, Ksp is 7.4×10-14 at 25°C. On the basis of this information and your ...
Introduction
... when they are placed in water, specifically ionic versus covalent compounds. One breaks apart in water, the other does not. Which one is more likely to be pulled apart by water molecules? Electrolytes are ionic and strong acid solutions (e.g., GatoradeTM); Nonelectrolytes are covalent compounds (e.g ...
... when they are placed in water, specifically ionic versus covalent compounds. One breaks apart in water, the other does not. Which one is more likely to be pulled apart by water molecules? Electrolytes are ionic and strong acid solutions (e.g., GatoradeTM); Nonelectrolytes are covalent compounds (e.g ...
WELCOME TO CLASS XII ORIENTATION IN CHEMISTRY SOME
... 3. Give reasons for the following: (i) Conc HNO3 turns yellow on exposure to sunlight. (ii) PCl5 behaves as an ionic species in solid state. Ans (i) Conc HNO3 decomposes to NO2 which is brown in colour& NO2 dissolves in HNO3 to it yellow. (ii) It exists as [PCl4]+[ PCl6]- in solid state. 4. What hap ...
... 3. Give reasons for the following: (i) Conc HNO3 turns yellow on exposure to sunlight. (ii) PCl5 behaves as an ionic species in solid state. Ans (i) Conc HNO3 decomposes to NO2 which is brown in colour& NO2 dissolves in HNO3 to it yellow. (ii) It exists as [PCl4]+[ PCl6]- in solid state. 4. What hap ...
CH 4 Notes
... The double arrow means that the reaction is significant in both directions. It indicates that there is a balance between the forward and reverse reactions. This balance produces a state of chemical equilibrium. ...
... The double arrow means that the reaction is significant in both directions. It indicates that there is a balance between the forward and reverse reactions. This balance produces a state of chemical equilibrium. ...
Chapter 24 Hoofstuk 24
... crystal chemically and chemically and describe the formation process(es) whereby this mineral is formed. Give the typical minerals that occur associated with your mineral and give the rock types in which it occurs. Give the most important uses for this mineral and state at least two locations in Sou ...
... crystal chemically and chemically and describe the formation process(es) whereby this mineral is formed. Give the typical minerals that occur associated with your mineral and give the rock types in which it occurs. Give the most important uses for this mineral and state at least two locations in Sou ...
Activity A: Minerals and Colour!
... Minerals are the building blocks of rocks and are made up of elements. They come in many different forms with some forming crystals with distinctive shapes. Rocks are made from grains of one or more mineral. Use the displays in the Albert Chapman Mineral Collection and the Planet of Minerals exhibit ...
... Minerals are the building blocks of rocks and are made up of elements. They come in many different forms with some forming crystals with distinctive shapes. Rocks are made from grains of one or more mineral. Use the displays in the Albert Chapman Mineral Collection and the Planet of Minerals exhibit ...
August 2007
... A student constructed an electrochemical cell as shown. The aqueous cell solutions had a concentration of 1 mol/L with respect to the metal ions present. The solution in one half-cell is initially an orange colour due to the mixture of the pale green Fe2+ ions and the orange Fe3+ ions. The other hal ...
... A student constructed an electrochemical cell as shown. The aqueous cell solutions had a concentration of 1 mol/L with respect to the metal ions present. The solution in one half-cell is initially an orange colour due to the mixture of the pale green Fe2+ ions and the orange Fe3+ ions. The other hal ...
III-nitride tunable cup-cavities supporting quasi whispering gallery modes from ultraviolet to infrared
... were at the maximum. Instead, the new modes come into play at the lower-energy side of the shifted PL band, because of changing the fluorescence coupling conditions. Between these boundaries, a set of the narrow lines can be found, whose positions are stable with increasing the temperature from 77 K ...
... were at the maximum. Instead, the new modes come into play at the lower-energy side of the shifted PL band, because of changing the fluorescence coupling conditions. Between these boundaries, a set of the narrow lines can be found, whose positions are stable with increasing the temperature from 77 K ...
Self-Assembly of the First Copper (II) Infinite 2D Network with Large
... containing copper atoms Cu1, Cu1A, Cu1B, Cu1C, and Cu1D are strictly parallel with each other. The distance between these two benzene rings is 10.00 Å, and the plane of copper atoms is at the middle of the two benzene ring planes; i.e., each copper atom is out of the benzene ring plane by distance o ...
... containing copper atoms Cu1, Cu1A, Cu1B, Cu1C, and Cu1D are strictly parallel with each other. The distance between these two benzene rings is 10.00 Å, and the plane of copper atoms is at the middle of the two benzene ring planes; i.e., each copper atom is out of the benzene ring plane by distance o ...
CHM 1033 Chemistry for Health Sciences
... CHM 1033 Chemistry for Health Sciences (Study and practice all questions. Select only 25 for your Home Work Assignment grade) Assignment I ...
... CHM 1033 Chemistry for Health Sciences (Study and practice all questions. Select only 25 for your Home Work Assignment grade) Assignment I ...
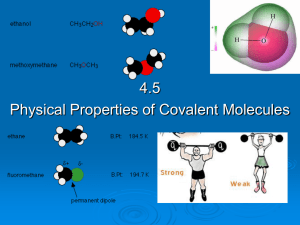
4.5 Physical properties of molecular covalent
... that are non-polar because they have the weakest intermolecular forces (van der Waals) between their molecules. ...
... that are non-polar because they have the weakest intermolecular forces (van der Waals) between their molecules. ...
Unit 2: Minerals
... Naturally occurring Inorganic Solid Composed of elements or compounds • Elements are composed of only one type of atom. • Compounds are 2 or more elements chemically combined ...
... Naturally occurring Inorganic Solid Composed of elements or compounds • Elements are composed of only one type of atom. • Compounds are 2 or more elements chemically combined ...
Volcanic Eruptions On a separate sheet of paper, explain what an
... Can you imagine a mineral crystal as big as a truck? Such crystals do exist. They sometimes are found in pegmatites, which are a type of mineral deposit. Pegmatites form from low-viscosity, watery magma. Viscosity refers to a fluid’s resistance to flow. High-viscosity magma is thick and flows slowly ...
... Can you imagine a mineral crystal as big as a truck? Such crystals do exist. They sometimes are found in pegmatites, which are a type of mineral deposit. Pegmatites form from low-viscosity, watery magma. Viscosity refers to a fluid’s resistance to flow. High-viscosity magma is thick and flows slowly ...
Critical Point Dryer
... First precursor gas (A Source) is introduced into the process chamber and produces a monolayer on the wafer surface. Then a second precursor gas (B Source) is introduced into the chamber, which reacts with the first precursor to produce a monolayer of film on the wafer surface. Separation of the pre ...
... First precursor gas (A Source) is introduced into the process chamber and produces a monolayer on the wafer surface. Then a second precursor gas (B Source) is introduced into the chamber, which reacts with the first precursor to produce a monolayer of film on the wafer surface. Separation of the pre ...
Minerals explained III—Rock forming non-silicates
... less common in terms of their abundance. Furthermore, there are some minerals that occur in a pure, native form as elements, primarily although not exclusively, metals, with some of the best known including gold, silver and copper. The latter mineral groupings, oxides, sulphides and native elements, ...
... less common in terms of their abundance. Furthermore, there are some minerals that occur in a pure, native form as elements, primarily although not exclusively, metals, with some of the best known including gold, silver and copper. The latter mineral groupings, oxides, sulphides and native elements, ...
Solution of the 1st Major Exam, Term 061, Version 000, all correct
... 18. Give the complete ionic equation for the reaction that occurs when aqueous solutions of lithium sulfide and copper (II) nitrate are mixed. A) 2 Li+(aq) + S2-(aq) + Cu2+(aq) + 2 NO3-(aq) → CuS(s) + 2 Li+(aq) + 2 NO3-(aq) B) 2Li+(aq) + SO42-(aq) + Cu2+(aq) + 2NO3-(aq) → CuSO4(s) + 2Li+(aq) + 2NO3- ...
... 18. Give the complete ionic equation for the reaction that occurs when aqueous solutions of lithium sulfide and copper (II) nitrate are mixed. A) 2 Li+(aq) + S2-(aq) + Cu2+(aq) + 2 NO3-(aq) → CuS(s) + 2 Li+(aq) + 2 NO3-(aq) B) 2Li+(aq) + SO42-(aq) + Cu2+(aq) + 2NO3-(aq) → CuSO4(s) + 2Li+(aq) + 2NO3- ...
Ligand Conformation Enforces Trigonal
... is EPR silent at room temperature and 77 K, thus suggesting a strongly antiferromagnetically coupled dinuclear compound. Description of the Crystal Structure. Table 1 lists the crystallographic data for complex 1, which crystallizes as green single crystals that belong to the triclinic system, space ...
... is EPR silent at room temperature and 77 K, thus suggesting a strongly antiferromagnetically coupled dinuclear compound. Description of the Crystal Structure. Table 1 lists the crystallographic data for complex 1, which crystallizes as green single crystals that belong to the triclinic system, space ...
Volcanic Eruptions
... Can you imagine a mineral crystal as big as a truck? Such crystals do exist. They sometimes are found in pegmatites, which are a type of mineral deposit. Pegmatites form from low-viscosity, watery magma. Viscosity refers to a fluid’s resistance to flow. High-viscosity magma is thick and flows slowly ...
... Can you imagine a mineral crystal as big as a truck? Such crystals do exist. They sometimes are found in pegmatites, which are a type of mineral deposit. Pegmatites form from low-viscosity, watery magma. Viscosity refers to a fluid’s resistance to flow. High-viscosity magma is thick and flows slowly ...
Chapter 3: Atoms, Elements, Minerals, Rocks
... Ionic substitution is the substitution of one ion for another in a compound. The bonding in most common minerals is ionic. Ionic substitution depends upon: Crystal structure; Ion size; - commonly expressed as ionic radius (distance from the center of the nucleus to the outermost shell of o ...
... Ionic substitution is the substitution of one ion for another in a compound. The bonding in most common minerals is ionic. Ionic substitution depends upon: Crystal structure; Ion size; - commonly expressed as ionic radius (distance from the center of the nucleus to the outermost shell of o ...
Crystallization

Crystallization is the (natural or artificial) process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction.