TextMineralProperties
... minerals and selected 10 of them to make up what we now called Mohs' Hardness Scale (Table 1.2). Talc, the softest of the ten, was assigned a hardness of 1; diamond, the hardest, was assigned a hardness of 10. All known minerals can be fitted between talc and diamond on this scale. Each mineral on M ...
... minerals and selected 10 of them to make up what we now called Mohs' Hardness Scale (Table 1.2). Talc, the softest of the ten, was assigned a hardness of 1; diamond, the hardest, was assigned a hardness of 10. All known minerals can be fitted between talc and diamond on this scale. Each mineral on M ...
to view
... electrons occupy anionic vacancies. These sites are called F centers. These electrons absorb energy from the visible region and transmits yellow colour. (ii) In the crystal of FeO, some of the Fe2+ cations are replaced by Fe3+ ions. Three Fe2+ ions are replaced by two Fe3+ ions to make up for the lo ...
... electrons occupy anionic vacancies. These sites are called F centers. These electrons absorb energy from the visible region and transmits yellow colour. (ii) In the crystal of FeO, some of the Fe2+ cations are replaced by Fe3+ ions. Three Fe2+ ions are replaced by two Fe3+ ions to make up for the lo ...
Equilibrium Part 2
... For the following reaction pressure changes would have NO effect on the equilibrium position. H2(g) + I2(g) ↔ 2 HI(g) Each side of the reaction has two moles of molecules. There is no way to either increase or reduce the number of particles. Therefore, in response to pressure changes, the equilibriu ...
... For the following reaction pressure changes would have NO effect on the equilibrium position. H2(g) + I2(g) ↔ 2 HI(g) Each side of the reaction has two moles of molecules. There is no way to either increase or reduce the number of particles. Therefore, in response to pressure changes, the equilibriu ...
analytical chemistry - Львівський національний медичний
... reagent run on filter paper with some drops (1-2) of solutions – arise a coloured spots. Requirements (demands) to analytical reactions: 1) reaction must run quickly, in practice – immediately; 2) reaction must accompanied with accordance (special) analytical effect; 3) reaction must be irreversible ...
... reagent run on filter paper with some drops (1-2) of solutions – arise a coloured spots. Requirements (demands) to analytical reactions: 1) reaction must run quickly, in practice – immediately; 2) reaction must accompanied with accordance (special) analytical effect; 3) reaction must be irreversible ...
Answers - University of Waterloo
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
Chapter 19.1 Balancing Redox Equations
... According to the collision theory of kinetics, which statement best describes the rate of a chemical reaction? a) All collisions result in a chemical reaction. b) The greater the difference in energy between the reactants and the transition state, the faster is the reaction. c) All collisions betwee ...
... According to the collision theory of kinetics, which statement best describes the rate of a chemical reaction? a) All collisions result in a chemical reaction. b) The greater the difference in energy between the reactants and the transition state, the faster is the reaction. c) All collisions betwee ...
Carefully detach the last page. It is the Data Sheet.
... © 2008 UNIVERSITY OF WATERLOO CHEM 13 NEWS EXAM / 3 ...
... © 2008 UNIVERSITY OF WATERLOO CHEM 13 NEWS EXAM / 3 ...
IONIC EQULIBRIUM
... Note: Exact treatment of this case is difficult to solve. So use this assumption in general cases. Also, degree of anion or cation will be much higher in the case of a salt of weak acid and weak base. This is because each of them gets hydrolysed, producing H+ and OH− ions. These ions combine to form ...
... Note: Exact treatment of this case is difficult to solve. So use this assumption in general cases. Also, degree of anion or cation will be much higher in the case of a salt of weak acid and weak base. This is because each of them gets hydrolysed, producing H+ and OH− ions. These ions combine to form ...
2014_S4_CHM_NORMAL (ALL)
... 53. Element X (atomic number 11) reacts with element Y (atomic number 16) to form an ionic compound. Each atom of X loses one electron and each atom of Y accepts two electrons to form a compound with formula X2Y. 54. Consider the following information: ...
... 53. Element X (atomic number 11) reacts with element Y (atomic number 16) to form an ionic compound. Each atom of X loses one electron and each atom of Y accepts two electrons to form a compound with formula X2Y. 54. Consider the following information: ...
Final Review 3-8 Answers_2
... a) S8(s) + 8 O2(g) 8 SO2(g) b) S(s) + O2(g) SO2(g) c) SO2(g) + H2O (l) H2SO3(aq) d) SO2(g) + ½ O2(g) SO3(g) 4. The experimental design of most directly determining the mass of solute in a specific volume of solution is called a) titration b) crystallization c) filtration d) distillation 5. I ...
... a) S8(s) + 8 O2(g) 8 SO2(g) b) S(s) + O2(g) SO2(g) c) SO2(g) + H2O (l) H2SO3(aq) d) SO2(g) + ½ O2(g) SO3(g) 4. The experimental design of most directly determining the mass of solute in a specific volume of solution is called a) titration b) crystallization c) filtration d) distillation 5. I ...
Full Text - The International Journal of Developmental Biology
... rather than pH 7.26 (the value reported by Aoba and Moreno (1987) to be the pH of the secretory stage enamel tissue fluid). The sensitivity of these experiments to pH is evident since the difference in binding affinity existing between the 25 kDa nascent amelogenin and its 20 kDa degradation product ...
... rather than pH 7.26 (the value reported by Aoba and Moreno (1987) to be the pH of the secretory stage enamel tissue fluid). The sensitivity of these experiments to pH is evident since the difference in binding affinity existing between the 25 kDa nascent amelogenin and its 20 kDa degradation product ...
COURSE CODE: CHM 291 COURSE TITLE: PRACTICAL CHEMISTRY (III) INORGANIC
... Laboratory Care and Waste Disposal Remember that the equipment you use in this laboratory will be used by many other students. Please leave the equipment and all workspaces as you wish to find them. After the end of the each lab, clean off your work area. Wash your glassware. When weighing any mater ...
... Laboratory Care and Waste Disposal Remember that the equipment you use in this laboratory will be used by many other students. Please leave the equipment and all workspaces as you wish to find them. After the end of the each lab, clean off your work area. Wash your glassware. When weighing any mater ...
Transport Processes: Momentum, Heat, and Mass
... 2. Heat transfer. In this fundamental process, we are concerned with the transfer of heat from one place to another; it occurs in the separation processes of drying, evaporation, distillation, and others. 3. Mass transfer. Here mass is being transferred from one phase to another distinct phase; the ...
... 2. Heat transfer. In this fundamental process, we are concerned with the transfer of heat from one place to another; it occurs in the separation processes of drying, evaporation, distillation, and others. 3. Mass transfer. Here mass is being transferred from one phase to another distinct phase; the ...
Sample pages
... 1.3.2.6.4. Fourier transforms of periodic distributions .. .. .. .. .. .. .. .. 1.3.2.6.5. The case of non-standard period lattices .. .. .. .. .. .. .. .. .. 1.3.2.6.6. Duality between periodization and sampling .. .. .. .. .. .. .. .. 1.3.2.6.7. The Poisson summation formula .. .. .. .. .. .. .. . ...
... 1.3.2.6.4. Fourier transforms of periodic distributions .. .. .. .. .. .. .. .. 1.3.2.6.5. The case of non-standard period lattices .. .. .. .. .. .. .. .. .. 1.3.2.6.6. Duality between periodization and sampling .. .. .. .. .. .. .. .. 1.3.2.6.7. The Poisson summation formula .. .. .. .. .. .. .. . ...
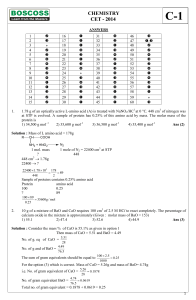
CHEMISTRY CET
... 2 NH3 + H2SO4 (NH4)2SO4 1 mole of ammonia requires 0.5 mole of H2SO4 and hence H2SO4 remain in the solution in the solution and hence the pH is very less decreasing order of pH is b > a > c ...
... 2 NH3 + H2SO4 (NH4)2SO4 1 mole of ammonia requires 0.5 mole of H2SO4 and hence H2SO4 remain in the solution in the solution and hence the pH is very less decreasing order of pH is b > a > c ...
QualGroupB - Back To Home Page
... 2. Removal of Any Contaminating Lead Cations: The solubility of PbCl2 is relatively high and some lead may not precipitate as PbCl2 during the group A analysis. If Pb2+ is present in your unknown, your Group B-D supernatant may contain some residual Pb2+ that will precipitate with your Group B catio ...
... 2. Removal of Any Contaminating Lead Cations: The solubility of PbCl2 is relatively high and some lead may not precipitate as PbCl2 during the group A analysis. If Pb2+ is present in your unknown, your Group B-D supernatant may contain some residual Pb2+ that will precipitate with your Group B catio ...
Unit 4 Chemical Kinetics and Chemical Equilibrium
... Le Chatelier’s Principle Reducing the volume (thereby increasing the partial pressures) of a gaseous system at equilibrium causes the reaction to shift in the direction that reduces the total number of moles of gas Increasing the volume (thereby decreasing the partial pressure) of a gaseous equ ...
... Le Chatelier’s Principle Reducing the volume (thereby increasing the partial pressures) of a gaseous system at equilibrium causes the reaction to shift in the direction that reduces the total number of moles of gas Increasing the volume (thereby decreasing the partial pressure) of a gaseous equ ...
DCY1B - Manonmaniam Sundaranar University
... steroids. This catalyst transfers the hydrogen atoms specifically to the 'cis' positions. (ii) It is an ideal catalyst used for catalysing hydrogenations at room temperature and pressure. (iii) Wilkinson's catalyst is more important in pharmaceutical and petrochemical industries for making specific ...
... steroids. This catalyst transfers the hydrogen atoms specifically to the 'cis' positions. (ii) It is an ideal catalyst used for catalysing hydrogenations at room temperature and pressure. (iii) Wilkinson's catalyst is more important in pharmaceutical and petrochemical industries for making specific ...
The three-dimensional structure of a photosystem II core complex
... form of crystal (Fig. 2a) appeared to be better ordered than the tube form and therefore this type of crystal was used for most of the image analysis. Fourier analysis of ...
... form of crystal (Fig. 2a) appeared to be better ordered than the tube form and therefore this type of crystal was used for most of the image analysis. Fourier analysis of ...
Unit- 5.pmd
... only occur if there is some possibility of chemical bonding between adsorbent and adsorbate. For example, oxygen is adsorbed on metals by virtue of oxide formation and hydrogen is adsorbed by transition metals due to hydride formation. (ii) Irreversibility: As chemisorption involves compound formati ...
... only occur if there is some possibility of chemical bonding between adsorbent and adsorbate. For example, oxygen is adsorbed on metals by virtue of oxide formation and hydrogen is adsorbed by transition metals due to hydride formation. (ii) Irreversibility: As chemisorption involves compound formati ...
Word - chemmybear.com
... B Notice this is at STP. 11.2 L = .50 moles, so the answer is ½ of 241.8 kJ/mol = 120.6 kJ B liquid gas is the greatest increase in entropy of the given examples. “A” and “D” are decreases in entropy. “C” has very little change in entropy. A H ; S + (liquid gases); so this will be product-fav ...
... B Notice this is at STP. 11.2 L = .50 moles, so the answer is ½ of 241.8 kJ/mol = 120.6 kJ B liquid gas is the greatest increase in entropy of the given examples. “A” and “D” are decreases in entropy. “C” has very little change in entropy. A H ; S + (liquid gases); so this will be product-fav ...
Solids Chemistry XII - The Gurukul Institute
... SOLUTIONS 1 MARK QUESTIONS Give two examples of gaseous solution. When would dissolving of solute in a solvent leads to liberation of heat energy? How is it that NaCl is soluble in water but not in benzene? What is the weight percent of a solution? State the unit of it in which it is expressed. ...
... SOLUTIONS 1 MARK QUESTIONS Give two examples of gaseous solution. When would dissolving of solute in a solvent leads to liberation of heat energy? How is it that NaCl is soluble in water but not in benzene? What is the weight percent of a solution? State the unit of it in which it is expressed. ...
Acrobat - chemmybear.com
... B Notice this is at STP. 11.2 L = .50 moles, so the answer is ½ of 241.8 kJ/mol = 120.6 kJ B liquid → gas is the greatest increase in entropy of the given examples. “A” and “D” are decreases in entropy. “C” has very little change in entropy. A ∆H −; ∆S + (liquid → gases); so this will be product- fa ...
... B Notice this is at STP. 11.2 L = .50 moles, so the answer is ½ of 241.8 kJ/mol = 120.6 kJ B liquid → gas is the greatest increase in entropy of the given examples. “A” and “D” are decreases in entropy. “C” has very little change in entropy. A ∆H −; ∆S + (liquid → gases); so this will be product- fa ...
The Crystal Structure of a Fusagenic Sperm Protein Reveals
... forms extensive contacts throughout the molecule with neighboring molecules. Many of these contacts also exist in the turns between helices R1 and R2 and between helices R4 and R5. Thus, the different contacts at the turns and termini probably result in the minor differences seen between the two mol ...
... forms extensive contacts throughout the molecule with neighboring molecules. Many of these contacts also exist in the turns between helices R1 and R2 and between helices R4 and R5. Thus, the different contacts at the turns and termini probably result in the minor differences seen between the two mol ...
Equilibrium notes (complete)
... There is more to spontaneity • Some endothermic reactions are spontaneous o Ammonium nitrate and water react spontaneously and absorb energy from the surroundings • Some exothermic reactions don’t go to completion o Equilibrium is established as the reverse endothermic reaction occurs Randomness is ...
... There is more to spontaneity • Some endothermic reactions are spontaneous o Ammonium nitrate and water react spontaneously and absorb energy from the surroundings • Some exothermic reactions don’t go to completion o Equilibrium is established as the reverse endothermic reaction occurs Randomness is ...
Crystallization

Crystallization is the (natural or artificial) process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction.