Design and Fabrication of a Superprism
... A superprism is an optical device which enhances the effects of a conventional prism in two ways: it disperses multiple wavelengths of light and allows for highly sensitive steering of a single wavelength beam over much wider angles than in a conventional prism. Photonic crystals offer an exciting n ...
... A superprism is an optical device which enhances the effects of a conventional prism in two ways: it disperses multiple wavelengths of light and allows for highly sensitive steering of a single wavelength beam over much wider angles than in a conventional prism. Photonic crystals offer an exciting n ...
Topic 1 - Coral Gables Senior High
... overturning the phlogiston theory. This is a good example of how the evolution of scientific ideas, such as how chemical change occurs, is based on the need for theories that can be tested by experiment. Where results are not compatible with the theory, a new theory must be put forward, which must t ...
... overturning the phlogiston theory. This is a good example of how the evolution of scientific ideas, such as how chemical change occurs, is based on the need for theories that can be tested by experiment. Where results are not compatible with the theory, a new theory must be put forward, which must t ...
2.1T - Introduction to Minerals Teacher
... Many textbooks have a fifth characteristic of minerals. They say that they are inorganic. This means that they did not come from a living organism. Some minerals form by organic means and inorganic means. For example, calcite forms both organically and inorganically. ...
... Many textbooks have a fifth characteristic of minerals. They say that they are inorganic. This means that they did not come from a living organism. Some minerals form by organic means and inorganic means. For example, calcite forms both organically and inorganically. ...
Holt Modern Chemistry Workbook: ch 11
... flat feet. The same force applied to a smaller area results in greater pressure. Atmospheric Pressure The atmosphere, which is the air that surrounds Earth, exerts pressure on Earth’s surface. This pressure is equivalent to a 1.03 kg mass sitting on every square centimeter of Earth. The resulting pr ...
... flat feet. The same force applied to a smaller area results in greater pressure. Atmospheric Pressure The atmosphere, which is the air that surrounds Earth, exerts pressure on Earth’s surface. This pressure is equivalent to a 1.03 kg mass sitting on every square centimeter of Earth. The resulting pr ...
An introduction to minerals and rocks under the microscope
... identification of igneous, metamorphic and sedimentary rocks, and the interpretation of the environment in which they formed. This free course introduces the polarising microscope, the main tool used to study minerals in rock thin sections, which remains the foundation of learning to recognise, char ...
... identification of igneous, metamorphic and sedimentary rocks, and the interpretation of the environment in which they formed. This free course introduces the polarising microscope, the main tool used to study minerals in rock thin sections, which remains the foundation of learning to recognise, char ...
Mineral Sheets compiled by Prof. J. Paquette
... Colour: variable. Often pinkish or light orange but may also be white, pale yellow, reddish, greenish, or gray. The variety AMAZONITE has a blue-green colour related to the presence of small amounts of Pb. Look-alikes: members of the plagioclase series (including albite). Most feldspars contain sodi ...
... Colour: variable. Often pinkish or light orange but may also be white, pale yellow, reddish, greenish, or gray. The variety AMAZONITE has a blue-green colour related to the presence of small amounts of Pb. Look-alikes: members of the plagioclase series (including albite). Most feldspars contain sodi ...
EDEXCEL A LeveL - Hodder Education
... 2 What steps should the students take to ensure that all the b) How much copper, in moles, combines with one mole of copper oxide is reduced to copper? oxygen in red copper oxide? 3 Start a spreadsheet program on a computer and open up c) What is the formula of red copper oxide? a new spreadsheet ...
... 2 What steps should the students take to ensure that all the b) How much copper, in moles, combines with one mole of copper oxide is reduced to copper? oxygen in red copper oxide? 3 Start a spreadsheet program on a computer and open up c) What is the formula of red copper oxide? a new spreadsheet ...
Example of Lab Notebook
... To an acidic aqueous solution, 5.2 mL of aniline was added producing the water soluble aniline hydrochloride. This was evident as the black oily liquid disappeared upon swirling. This was followed by the addition of 6.3 mL of acetic anhydride which produced some precipitation. Following the addition ...
... To an acidic aqueous solution, 5.2 mL of aniline was added producing the water soluble aniline hydrochloride. This was evident as the black oily liquid disappeared upon swirling. This was followed by the addition of 6.3 mL of acetic anhydride which produced some precipitation. Following the addition ...
([Cu(NH3)4](MnO4)2)
... tetramminecopper-complex cation) [6] (Eqn. 1). An increase in temperature favorably affects the formation of 2, since the solubility of 1 increases; therefore, the amount of the dissociated tetraamminecopper(2 ) ion, as well as the concentrations of the free (released) copper(2 ) ions and NH3 , al ...
... tetramminecopper-complex cation) [6] (Eqn. 1). An increase in temperature favorably affects the formation of 2, since the solubility of 1 increases; therefore, the amount of the dissociated tetraamminecopper(2 ) ion, as well as the concentrations of the free (released) copper(2 ) ions and NH3 , al ...
!GLG 101-Illustrated Vocabulary-Chapter 3 !Igneous Minerals and
... -occurs as a subducted slab of crust moves down a subduction zone. Those minerals with the lowest melting temperatures melt first. pegmatite -is a very coarse grained igneous rock. The crystals within a pegmatite are larger than one half inch. -pegmatite-1 -pegmatite-2 perlite -is a volcanic glass ...
... -occurs as a subducted slab of crust moves down a subduction zone. Those minerals with the lowest melting temperatures melt first. pegmatite -is a very coarse grained igneous rock. The crystals within a pegmatite are larger than one half inch. -pegmatite-1 -pegmatite-2 perlite -is a volcanic glass ...
At equilibrium
... relative amounts of CaO and CaCO3 present. K increases with T (=> endothermic reaction) and exceeds 1 at above 1000oC (--> equilm pCO2 > 1 atm). It is then simply necessary to allow the CO2 to escape to obtain more CaO from the CaCO3. ...
... relative amounts of CaO and CaCO3 present. K increases with T (=> endothermic reaction) and exceeds 1 at above 1000oC (--> equilm pCO2 > 1 atm). It is then simply necessary to allow the CO2 to escape to obtain more CaO from the CaCO3. ...
UvA-DARE (Digital Academic Repository) Rotational symmetry
... for B ⊥ I to be broader than the curves for B || I. However, we observe the reverse (see Fig. 3a,b). Moreover, the anisotropy is still present at T/Tc = 0.1 and is much larger (of the order of 300%, see Fig. 4) than can be expected on the basis of flux flow. In order to further rule out the influ ...
... for B ⊥ I to be broader than the curves for B || I. However, we observe the reverse (see Fig. 3a,b). Moreover, the anisotropy is still present at T/Tc = 0.1 and is much larger (of the order of 300%, see Fig. 4) than can be expected on the basis of flux flow. In order to further rule out the influ ...
0829.PDF
... steel frame. Dynamic tests were conducted by placing the load frame into a compression Hopkinson bar apparatus. The loading pulse could then be controlled by the striker bar length and velocity. A 6.4 mm thick loading pin transfers the loading pulse from the Hopkinson bar to a 1018 steel bar that sp ...
... steel frame. Dynamic tests were conducted by placing the load frame into a compression Hopkinson bar apparatus. The loading pulse could then be controlled by the striker bar length and velocity. A 6.4 mm thick loading pin transfers the loading pulse from the Hopkinson bar to a 1018 steel bar that sp ...
Physical and Chemical equilibrium
... Dissolution of gasses in water is always exothermic. As dissolution is spontaneous( takes place on its own) ∆G = -ve, entropy decreases in the process of dissolution ∆S = -ve. Therefore ∆H has be negative i.e. exothermic. The amount of gas dissolved is given by Henry’s law Henry’s law The law states ...
... Dissolution of gasses in water is always exothermic. As dissolution is spontaneous( takes place on its own) ∆G = -ve, entropy decreases in the process of dissolution ∆S = -ve. Therefore ∆H has be negative i.e. exothermic. The amount of gas dissolved is given by Henry’s law Henry’s law The law states ...
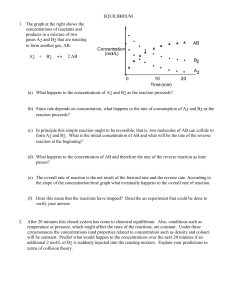
EQUILIBRIUM - SCH4U1-CCVI
... be made about the equilibrium concentrations in other solutions. To prepare a solution with a known concentration of the coloured complex, a dilute solution of thiocyanate ion is reacted with a concentrated solution of iron (III) ion. It may be assumed that the large excess of iron (III) ion causes ...
... be made about the equilibrium concentrations in other solutions. To prepare a solution with a known concentration of the coloured complex, a dilute solution of thiocyanate ion is reacted with a concentrated solution of iron (III) ion. It may be assumed that the large excess of iron (III) ion causes ...
1aUnit Two Handouts - Dunmore High School
... DECISION TREE: Should a formula be written as ions? Is the substance an acid (formula begins with H), a base (contains hydroxide ion), a salt (cation anion) or other? If it is an acid, go to A. If it is a base, go to B. If it is a salt, go to S. If it is an other, do not write it as ions. Example: Z ...
... DECISION TREE: Should a formula be written as ions? Is the substance an acid (formula begins with H), a base (contains hydroxide ion), a salt (cation anion) or other? If it is an acid, go to A. If it is a base, go to B. If it is a salt, go to S. If it is an other, do not write it as ions. Example: Z ...
Unit V The Mole
... If 1 L of nitrogen reacts with 3 L of hydrogen to form ammonia, then its formula is __________ If 2 L of hydrogen reacts with 1 L of oxygen to form water, then its formula is __________ ...
... If 1 L of nitrogen reacts with 3 L of hydrogen to form ammonia, then its formula is __________ If 2 L of hydrogen reacts with 1 L of oxygen to form water, then its formula is __________ ...
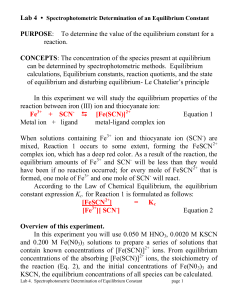
PURPOSE: To determine the value of the equilibrium constant for a
... There is a universal tendency of metal ions to form complexes with negatively charged ions (e.g. SCN -) and with neutral molecules (e.g., water, H2O, and ammonia, NH3) that have lone pairs of electrons (nonbonding pairs). This tendency can be understood as the result of the attractive force between ...
... There is a universal tendency of metal ions to form complexes with negatively charged ions (e.g. SCN -) and with neutral molecules (e.g., water, H2O, and ammonia, NH3) that have lone pairs of electrons (nonbonding pairs). This tendency can be understood as the result of the attractive force between ...
Combo 4.14.2 Inside Earth
... Identifying Minerals • Crystal – A solid in which the atoms are arranged in a pattern that repeats over & over • Streak – The color of a mineral’s powder. • Luster – The way a mineral reflects light from its ...
... Identifying Minerals • Crystal – A solid in which the atoms are arranged in a pattern that repeats over & over • Streak – The color of a mineral’s powder. • Luster – The way a mineral reflects light from its ...
Document
... What is the maximum amount of O2 in grams that can be obtained from 2.00 x102 g of nitroglycerin? Calculate the percent yield in this reaction if the amount of O2 generated is found to be 6.55 g. ...
... What is the maximum amount of O2 in grams that can be obtained from 2.00 x102 g of nitroglycerin? Calculate the percent yield in this reaction if the amount of O2 generated is found to be 6.55 g. ...
General Concepts of Chemical Equilibrium
... In a chemical reaction, as reactants start to react products start to form. Therefore, reactants continuously decrease and products continuously increase till a point is reached where eventually no change in concentrations can be detected. This is the point of equilibrium which is a point where the ...
... In a chemical reaction, as reactants start to react products start to form. Therefore, reactants continuously decrease and products continuously increase till a point is reached where eventually no change in concentrations can be detected. This is the point of equilibrium which is a point where the ...
Student Review Packet
... NOTE: Graph should have “pH” as the vertical axis and “added base” as the horizontal axis. The graph should be in a “double S” shape. The middle of the lower part of the “first S” indicates the point of maximum buffering of the first buffering zone where [H2A] / [HA-] = 1. The middle of the “first S ...
... NOTE: Graph should have “pH” as the vertical axis and “added base” as the horizontal axis. The graph should be in a “double S” shape. The middle of the lower part of the “first S” indicates the point of maximum buffering of the first buffering zone where [H2A] / [HA-] = 1. The middle of the “first S ...
15equil1pp
... In this case you get to the equilibrium position quicker but with a reduced yield because the increased temperature moves the equilibrium to the left. In many industrial processes a compromise temperature is used (see Haber and Contact Processes). To reduce the problem one must look for a way of inc ...
... In this case you get to the equilibrium position quicker but with a reduced yield because the increased temperature moves the equilibrium to the left. In many industrial processes a compromise temperature is used (see Haber and Contact Processes). To reduce the problem one must look for a way of inc ...
Crystallization

Crystallization is the (natural or artificial) process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction.









2)](http://s1.studyres.com/store/data/015968611_1-56df287e8435abc2be6b0a2948d2417f-300x300.png)