Chemistry II - Mr. Dougan`s Wonderful World of Chemistry
... 8. Determine and record the experimental enthalpy of neutralization on the data table. Part Two: Heat of Neutralization of Hydrochloric Acid and Ammonia 1. Wash your calorimeter with water, dry with a paper towel, and reassemble. Mass the entire calorimeter to the nearest hundredth of a gram. 2. Add ...
... 8. Determine and record the experimental enthalpy of neutralization on the data table. Part Two: Heat of Neutralization of Hydrochloric Acid and Ammonia 1. Wash your calorimeter with water, dry with a paper towel, and reassemble. Mass the entire calorimeter to the nearest hundredth of a gram. 2. Add ...
General and Inorganic Chemistry – Laboratory Techniques
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...
Minerals - GVLibraries
... …as we explore the fascinating topic of minerals. Graphic Transition - What are Minerals? The mineral turquoise, in this piece of jewelry,… …and the mineral gypsum, used to make sheetrock, are just two examples of the more than 3,000 known minerals. Surprisingly, though, over 90% of the rocks in the ...
... …as we explore the fascinating topic of minerals. Graphic Transition - What are Minerals? The mineral turquoise, in this piece of jewelry,… …and the mineral gypsum, used to make sheetrock, are just two examples of the more than 3,000 known minerals. Surprisingly, though, over 90% of the rocks in the ...
doc - Dartmouth College
... The approach here is to use the relation K = e – Greaxn/RT and the thermodynamic data provided with the examination to calculate a numerical value for the equilibrium constant K. This requires that we determine Goreaxn = Horeaxn – T Soreaxn . From the thermodynamic data provided, we can calculat ...
... The approach here is to use the relation K = e – Greaxn/RT and the thermodynamic data provided with the examination to calculate a numerical value for the equilibrium constant K. This requires that we determine Goreaxn = Horeaxn – T Soreaxn . From the thermodynamic data provided, we can calculat ...
Reaction Rates/Chemical Kinetics
... In this reaction, SO2 and O2 are placed in a container. Initially, the forward reaction proceeds and SO3 is produced. The rate of the forward reaction is much greater than the rate of the reverse reaction. As SO3 builds up, it starts to decompose into SO2 and O2. The rate of the forward reaction is ...
... In this reaction, SO2 and O2 are placed in a container. Initially, the forward reaction proceeds and SO3 is produced. The rate of the forward reaction is much greater than the rate of the reverse reaction. As SO3 builds up, it starts to decompose into SO2 and O2. The rate of the forward reaction is ...
sch103manual - university of nairobi staff profiles
... looks at the chemical equilibrium and part four covers ionic equilibrium. It is a one year course which comprises both theoretical and practical components. The theory component will be sent to you as a full unit, with the prescribed accompanying text-book(s) and the practical unit. The practical se ...
... looks at the chemical equilibrium and part four covers ionic equilibrium. It is a one year course which comprises both theoretical and practical components. The theory component will be sent to you as a full unit, with the prescribed accompanying text-book(s) and the practical unit. The practical se ...
Chapter 15. Chemical Equilibrium
... Equilibria in which all reactants and products are present in the same phase are called homogeneous equilibria. • Equilibria in which one or more reactants or products are present in a different phase are called heterogeneous equilibria. • Consider the equilibrium established when solid lead(II) chl ...
... Equilibria in which all reactants and products are present in the same phase are called homogeneous equilibria. • Equilibria in which one or more reactants or products are present in a different phase are called heterogeneous equilibria. • Consider the equilibrium established when solid lead(II) chl ...
20. Chemical Equilibrium
... conversion of reactants into products is often incomplete in chemical reactions. This is the case no matter how long the reaction is allowed to continue. As a reaction progresses, the concentrations of the reactants decrease, while the concentrations of the products increase. Eventually, a state is ...
... conversion of reactants into products is often incomplete in chemical reactions. This is the case no matter how long the reaction is allowed to continue. As a reaction progresses, the concentrations of the reactants decrease, while the concentrations of the products increase. Eventually, a state is ...
Chemistry booklet
... , since it contains both a positive electric ‘pole’ (δ+) and a negative electric ‘pole’ (δ-), the symbol δ ‘delta’ means a ‘small amount’ ) . ...
... , since it contains both a positive electric ‘pole’ (δ+) and a negative electric ‘pole’ (δ-), the symbol δ ‘delta’ means a ‘small amount’ ) . ...
sample chapter
... Many chemical reactions and virtually all biological processes take place in an aqueous environment. Therefore, it is important to understand the properties of different substances in solution with water. To start with, what exactly is a solution? A solution is a homogeneous mixture of two or more s ...
... Many chemical reactions and virtually all biological processes take place in an aqueous environment. Therefore, it is important to understand the properties of different substances in solution with water. To start with, what exactly is a solution? A solution is a homogeneous mixture of two or more s ...
Solutions for Chapter 8 End-of-Chapter Problems
... (a) Ice cream melting will decrease the organization of the system. Rather than being in identifiable scoops of ice cream perched securely on a cone, there now will be liquid ice cream dripping on the outside of the cone, onto your hands, and possibly onto your clothes as well. (b) Students entering ...
... (a) Ice cream melting will decrease the organization of the system. Rather than being in identifiable scoops of ice cream perched securely on a cone, there now will be liquid ice cream dripping on the outside of the cone, onto your hands, and possibly onto your clothes as well. (b) Students entering ...
SCH4U Exam Review
... 28. Copper(I) chloride has ksp = 1.9x10-7. Calculate the molar solubility of CuCl in (a) pure water (b) 0.010 M HCl solution (c) 0.10 M HCl solution (d) 0.10 M CaCl2 solution. ANS: 4.4x10-4, 1.9x10-5, 1.9x10-6, 9.5x10-7 29. What is the common ion effect? How does Le Chatelier’s principle explain it? ...
... 28. Copper(I) chloride has ksp = 1.9x10-7. Calculate the molar solubility of CuCl in (a) pure water (b) 0.010 M HCl solution (c) 0.10 M HCl solution (d) 0.10 M CaCl2 solution. ANS: 4.4x10-4, 1.9x10-5, 1.9x10-6, 9.5x10-7 29. What is the common ion effect? How does Le Chatelier’s principle explain it? ...
NICKEL(II) PINCER COMPLEXES SUPPORTED BY 2,6
... The starting material of the ligand synthesis, 2,6-dicarboxaldehye was prepared from 2,6-dimethanol pyridine through oxidation with SeO2/Dioxane (Figure 9). Then, tolualdehyde was stirred at room temperature for 5 h with 4-methylacetophenone and NaOH in EtOH/H2O to form 1,3-bis(4-tolyl)-2-propen-1-o ...
... The starting material of the ligand synthesis, 2,6-dicarboxaldehye was prepared from 2,6-dimethanol pyridine through oxidation with SeO2/Dioxane (Figure 9). Then, tolualdehyde was stirred at room temperature for 5 h with 4-methylacetophenone and NaOH in EtOH/H2O to form 1,3-bis(4-tolyl)-2-propen-1-o ...
Rocks - Mid-Georgia Gem and Mineral Society
... Almost every mineral has an inherent streak color, no matter what color the actual mineral is. For example, Calcite occurs in many different colors, shapes, and varieties. But every single variety of Calcite has a white streak. A streak is useful in distinguishing two minerals with the same color bu ...
... Almost every mineral has an inherent streak color, no matter what color the actual mineral is. For example, Calcite occurs in many different colors, shapes, and varieties. But every single variety of Calcite has a white streak. A streak is useful in distinguishing two minerals with the same color bu ...
Chapter 9: Non-aqueous media
... A protic solvent undergoes self-ionization (see Section 7.2) to provide protons which are solvated. If it undergoes selfionization, an aprotic solvent does so without the formation of protons. ...
... A protic solvent undergoes self-ionization (see Section 7.2) to provide protons which are solvated. If it undergoes selfionization, an aprotic solvent does so without the formation of protons. ...
Spectroscopy of evaporites
... Light absorption occurs when anion groups, such as S04, C0 3 , and water molecule are present. Since evaporite minerals contain these compounds, it is theoretically possible to identify the specific evaporite mineral through spectra matching with the known spectra libraries, provided that the evapor ...
... Light absorption occurs when anion groups, such as S04, C0 3 , and water molecule are present. Since evaporite minerals contain these compounds, it is theoretically possible to identify the specific evaporite mineral through spectra matching with the known spectra libraries, provided that the evapor ...
Construction of Porous Solids from Hydrogen
... of BTC (BTCH3) (0.20 g, 0.95 mmol) was placed in a stainless steel vessel, which was sealed and placed in a programmable furnace. The mixture was heated to 140 °C at 5 °C/min and held at that temperature for 24 h, then cooled at 0.1 °C/min to 120 °C and held for 5 h, followed by further cooling at t ...
... of BTC (BTCH3) (0.20 g, 0.95 mmol) was placed in a stainless steel vessel, which was sealed and placed in a programmable furnace. The mixture was heated to 140 °C at 5 °C/min and held at that temperature for 24 h, then cooled at 0.1 °C/min to 120 °C and held for 5 h, followed by further cooling at t ...
The Extended and Eccentric E-DNA Structure Induced by Cytosine
... of the modified cytosines away from the helix axis than in A-DNA, as is evident from the more negative x-displacement. In addition, the shortened distance between phosphates of the modified (5.7 ± 0.2 Å) versus unmodified base pairs (6.5 ± 0.3 Å) is comparable to differences between ADNA (5.8 ± 0.2 ...
... of the modified cytosines away from the helix axis than in A-DNA, as is evident from the more negative x-displacement. In addition, the shortened distance between phosphates of the modified (5.7 ± 0.2 Å) versus unmodified base pairs (6.5 ± 0.3 Å) is comparable to differences between ADNA (5.8 ± 0.2 ...
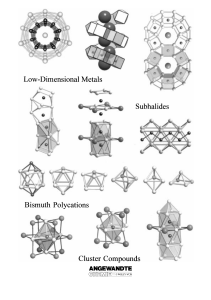
From the Metal to the Molecule
... intermetallic phases with bismuth. Previous studies suggest that this is a basic requirement for the formation of the corresponding subhalides. Consequently, we can speak of the partial oxidation of intermetallic phases. 2) The electronegativities of these transition metal elements hardly differ fro ...
... intermetallic phases with bismuth. Previous studies suggest that this is a basic requirement for the formation of the corresponding subhalides. Consequently, we can speak of the partial oxidation of intermetallic phases. 2) The electronegativities of these transition metal elements hardly differ fro ...
5 SURFACE CHEMISTRY CATEGORY
... freezing point by 7.5°C? The freezing point depression constant, Kf , for water is 1.86 K kg mol–1. Assume van’t Hoff factor for NaCl is 1.87. 8. 18 g of glucose, C6H12O6 (Molar Mass = 180 g mol–1) is dissolved in 1 kg of water in a sauce pan. At what temperature will this solution boil? 9.Determine ...
... freezing point by 7.5°C? The freezing point depression constant, Kf , for water is 1.86 K kg mol–1. Assume van’t Hoff factor for NaCl is 1.87. 8. 18 g of glucose, C6H12O6 (Molar Mass = 180 g mol–1) is dissolved in 1 kg of water in a sauce pan. At what temperature will this solution boil? 9.Determine ...
chapter 16
... You must check validity by plugging "x" over original concentration. It must be less than 5% of the original concentration to be valid. 2. If "x" is necessary, then see if the problem may be a perfect square and thus, ease the steps of solving. (Sometimes you must use the quadratic formula!) 3. If n ...
... You must check validity by plugging "x" over original concentration. It must be less than 5% of the original concentration to be valid. 2. If "x" is necessary, then see if the problem may be a perfect square and thus, ease the steps of solving. (Sometimes you must use the quadratic formula!) 3. If n ...
Appendices and Glossary
... If someone told you that she was “six”, you might have a little trouble deciding what was meant. That person could be six years old, but if she were a college student, that would probably not be correct. She could weigh six tons or be six inches tall, but probably not. She is more likely six feet ta ...
... If someone told you that she was “six”, you might have a little trouble deciding what was meant. That person could be six years old, but if she were a college student, that would probably not be correct. She could weigh six tons or be six inches tall, but probably not. She is more likely six feet ta ...
NLRP3 inflammasome activation by crystal structures
... thought that the different crystals act via common mechanisms to activate NLRP3. Lysosome rupture and cathepsin B leakage is one of the proposed models. During phagocytosis, the phagocytosed material is incorporated in a huge vesicle called phagosome. Then the phagosome fuses with lysosome to obtain ...
... thought that the different crystals act via common mechanisms to activate NLRP3. Lysosome rupture and cathepsin B leakage is one of the proposed models. During phagocytosis, the phagocytosed material is incorporated in a huge vesicle called phagosome. Then the phagosome fuses with lysosome to obtain ...
Oxidized and Reduced Azotobacter Vinelandii
... at near atomic resolution. The reduced state of the [3Fe-4S]0 cluster is established by single-crystal EPR following data collection. Redundant structures are refined to establish the reproducibility and accuracy of the results for both oxidation states. The structure of the [4Fe-4S]2+ cluster in fo ...
... at near atomic resolution. The reduced state of the [3Fe-4S]0 cluster is established by single-crystal EPR following data collection. Redundant structures are refined to establish the reproducibility and accuracy of the results for both oxidation states. The structure of the [4Fe-4S]2+ cluster in fo ...
Crystallization

Crystallization is the (natural or artificial) process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction.