ism ismismismismismrapidrevisionquestionsismismismismismism
... Number of atoms in primitive cube ( Rank) = 1 Number of atoms in BCC (Rank) = 2 Number of atoms in FCC (Rank) = 4 State a feature to distinguish (i) Metallic solid from ionic solid (ii) Covalent solid from molecular solid Constituent particles in metallic solids are positive metal ions (kernels) and ...
... Number of atoms in primitive cube ( Rank) = 1 Number of atoms in BCC (Rank) = 2 Number of atoms in FCC (Rank) = 4 State a feature to distinguish (i) Metallic solid from ionic solid (ii) Covalent solid from molecular solid Constituent particles in metallic solids are positive metal ions (kernels) and ...
Chem12 Buffer/Titration : Probs
... 28) Which one of the following equations contains the conjugate acidbase pair from which a buffer solution can be prepared ? a) HI(aq) + H2O(l) <-> H3O +(aq) + I-(aq) b) HBr(aq) + H2O(l) <-> H3O + (aq) + Br-(aq) c) H2SO 4(aq) + H2O(l) <-> H3O +(aq) + HSO4-(aq) d) H2CO3(aq) + H2O(l) <-> H3O+(aq) + HC ...
... 28) Which one of the following equations contains the conjugate acidbase pair from which a buffer solution can be prepared ? a) HI(aq) + H2O(l) <-> H3O +(aq) + I-(aq) b) HBr(aq) + H2O(l) <-> H3O + (aq) + Br-(aq) c) H2SO 4(aq) + H2O(l) <-> H3O +(aq) + HSO4-(aq) d) H2CO3(aq) + H2O(l) <-> H3O+(aq) + HC ...
Pirogov National Medical Univercity of Vinnitsa
... purification of substances as the work with contaminated reagents may lead to error in results. Clean substances that are used as medicinal products, determined by the State Pharmacopoeia of Ukraine and, therefore, pharmacists need to know basic purification and methods of identifying substances. 2. ...
... purification of substances as the work with contaminated reagents may lead to error in results. Clean substances that are used as medicinal products, determined by the State Pharmacopoeia of Ukraine and, therefore, pharmacists need to know basic purification and methods of identifying substances. 2. ...
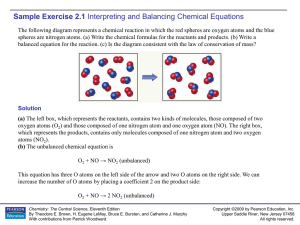
Sample Exercise 2.1
... Write the balanced equation for the reaction that occurs when methanol, CH 3OH(l), is burned in air. Solution When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In t ...
... Write the balanced equation for the reaction that occurs when methanol, CH 3OH(l), is burned in air. Solution When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In t ...
In_Class_Practice Chapter 17 PreAP
... Calculate Keq for this equilibrium using the data [NOBr] = 0.0474 mol/L, [NO] = 0.312 mol/L, and [Br2] = 0.259 mol/L. Practice Problems 3. The following is the chemical equation for the decomposition of formamide. HCONH2(g) NH3(g) + CO(g) Calculate Keq using the equilibrium data [HCONH2] = 0.0637 ...
... Calculate Keq for this equilibrium using the data [NOBr] = 0.0474 mol/L, [NO] = 0.312 mol/L, and [Br2] = 0.259 mol/L. Practice Problems 3. The following is the chemical equation for the decomposition of formamide. HCONH2(g) NH3(g) + CO(g) Calculate Keq using the equilibrium data [HCONH2] = 0.0637 ...
ESO - ENCIGA
... tested by subsequent investigation and can be modified by its results. Science does not give statements of absolute eternal truth, it only provides theories. We know that those theories will probably be refined in the future, and some of them may even be discarded in favour of theories that make mor ...
... tested by subsequent investigation and can be modified by its results. Science does not give statements of absolute eternal truth, it only provides theories. We know that those theories will probably be refined in the future, and some of them may even be discarded in favour of theories that make mor ...
Review Answers - cloudfront.net
... getting more disordered and therefore entropy is increased. ΔS° is positive b. What change, if any, will occur in ΔG° for the reaction as the temperature is increased? Explain your reasoning in terms of thermodynamics principles. Since when Temp is increased there are more molecules of PCl3 and Cl2, ...
... getting more disordered and therefore entropy is increased. ΔS° is positive b. What change, if any, will occur in ΔG° for the reaction as the temperature is increased? Explain your reasoning in terms of thermodynamics principles. Since when Temp is increased there are more molecules of PCl3 and Cl2, ...
Chapter 19 Chemical Thermodynamics
... Entropy changes for a reaction can be estimated in a manner analogous to that by which H is estimated: S° = nS°(products) - mS°(reactants) where n and m are the coefficients in the Chemical ...
... Entropy changes for a reaction can be estimated in a manner analogous to that by which H is estimated: S° = nS°(products) - mS°(reactants) where n and m are the coefficients in the Chemical ...
FOURTH GRADE MINERALS - Math/Science Nucleus
... Cleavage is a regular breakage that follows the atomic structure of a mineral. Cleavage results in smooth, planar surfaces. Different minerals may have one, two, three, four, or six cleavages. C. HARDNESS - The mineral’s resistance to scratching. It is controlled by the strength of atomic bonds with ...
... Cleavage is a regular breakage that follows the atomic structure of a mineral. Cleavage results in smooth, planar surfaces. Different minerals may have one, two, three, four, or six cleavages. C. HARDNESS - The mineral’s resistance to scratching. It is controlled by the strength of atomic bonds with ...
Chapter 4 Solution Chemistry
... aqueous solution, in which the solvent is water. • In this chapter, we’ll see how some types of chemical reactions take place and how we can organize chemical reactions into different types. Most of these reactions will take place in aqueous solutions. ...
... aqueous solution, in which the solvent is water. • In this chapter, we’ll see how some types of chemical reactions take place and how we can organize chemical reactions into different types. Most of these reactions will take place in aqueous solutions. ...
Practice Exam I FR Answers and Explanations
... Cd changes oxidation states from 0 to +2—thus, it is oxidized. Whatever species is oxidized is known as the reducing agent. (c) At a higher temperature, how would the cell potential change? Explain questions such as this with mathematical formulas if at all possible. There are two equations that all ...
... Cd changes oxidation states from 0 to +2—thus, it is oxidized. Whatever species is oxidized is known as the reducing agent. (c) At a higher temperature, how would the cell potential change? Explain questions such as this with mathematical formulas if at all possible. There are two equations that all ...
regents chemistry midterm - irondequoit 2014_entire exam w key
... the number of completely filled principal energy levels. [1pt] the type of bond that forms when its chemically combines with chlorine. [1pt] the number of protons in its nucleus. [1pt] the electron dot diagram (Lewis dot diagram) for an atom of this element. [1pt] the specific ion it will most likel ...
... the number of completely filled principal energy levels. [1pt] the type of bond that forms when its chemically combines with chlorine. [1pt] the number of protons in its nucleus. [1pt] the electron dot diagram (Lewis dot diagram) for an atom of this element. [1pt] the specific ion it will most likel ...
Solubility Solubility is defined as the amount of solute that will
... final time - initial time min This will give us a average rate because we will find that often the rate will change as the reaction proceeds. Also you might observe that the negative sign in front of the equation makes the value positive, as required by the definitition of rate. The rate is the expe ...
... final time - initial time min This will give us a average rate because we will find that often the rate will change as the reaction proceeds. Also you might observe that the negative sign in front of the equation makes the value positive, as required by the definitition of rate. The rate is the expe ...
Bulgarian Chemical Communications, Volume 41, Number 4 (pp
... organic matter. The coloured waste water is directly discharged into the rivers and other water ways. Traditional methods like adsorption on activated carbon, liquid-liquid extraction, ion-exchange, air or stream stripping, etc., are ineffective on refractory and non-volatile pollutants and have ano ...
... organic matter. The coloured waste water is directly discharged into the rivers and other water ways. Traditional methods like adsorption on activated carbon, liquid-liquid extraction, ion-exchange, air or stream stripping, etc., are ineffective on refractory and non-volatile pollutants and have ano ...
stoichiometric relationships - Assets
... An ideal gas is used to model the behaviour of real gases.Two assumptions made when defining an ideal gas are that the molecules themselves have no volume and that no forces exist between them (except when they collide). Gases deviate most from ideal behaviour at high pressure and low temperature (w ...
... An ideal gas is used to model the behaviour of real gases.Two assumptions made when defining an ideal gas are that the molecules themselves have no volume and that no forces exist between them (except when they collide). Gases deviate most from ideal behaviour at high pressure and low temperature (w ...
Amount of Substance
... proton and 1 electron. Since the mass of an electron is negligible compared to that of a proton or a neutron, the hydrogen atom has only 1/12 the mass of a carbon atom; therefore the relative atomic mass of hydrogen is 1. Similarly, the relative mass of an oxygen atom, which contains 8 protons, 8 ne ...
... proton and 1 electron. Since the mass of an electron is negligible compared to that of a proton or a neutron, the hydrogen atom has only 1/12 the mass of a carbon atom; therefore the relative atomic mass of hydrogen is 1. Similarly, the relative mass of an oxygen atom, which contains 8 protons, 8 ne ...
October 24 AR 1:34 * 1:49 pm
... Salt Crystal lab – first observations. Notes on compound micro type. Write your summary in full sentences and list one thing you learned ...
... Salt Crystal lab – first observations. Notes on compound micro type. Write your summary in full sentences and list one thing you learned ...
Net ionic equation
... A base is a substance that forms OH- ion when added to water (Arrhenius definition). A strong soluble base is a soluble hydroxide compound that completely dissociates when added to water. An insoluble base is an insoluble hydroxide compound. There are also a few substances that act as weak bases in ...
... A base is a substance that forms OH- ion when added to water (Arrhenius definition). A strong soluble base is a soluble hydroxide compound that completely dissociates when added to water. An insoluble base is an insoluble hydroxide compound. There are also a few substances that act as weak bases in ...
Biochem. J. (2006) 395, 457–462
... three substrate binding subsites − 1, + 1 and + 2 (Figure 3; subsite nomenclature adapted from Davies et al. [26] with cleavage taking place between − 1 and + 1, and the β-fructofuranosyl unit undergoing catalysis placed at subsite − 1). For all six independent molecules within the asymmetric unit, ...
... three substrate binding subsites − 1, + 1 and + 2 (Figure 3; subsite nomenclature adapted from Davies et al. [26] with cleavage taking place between − 1 and + 1, and the β-fructofuranosyl unit undergoing catalysis placed at subsite − 1). For all six independent molecules within the asymmetric unit, ...
stoichiometry - einstein classes
... EQUIVALENT CONCEPT It is based on law of equivalence which is explained as follows : Law of chemical equivalents : In a chemical reaction the equivalents of all the species (reactants or products) are equal to each other provided none of these compounds is in excess. N1V1 = N2V2 (when normalities an ...
... EQUIVALENT CONCEPT It is based on law of equivalence which is explained as follows : Law of chemical equivalents : In a chemical reaction the equivalents of all the species (reactants or products) are equal to each other provided none of these compounds is in excess. N1V1 = N2V2 (when normalities an ...
A flask contains 0
... o Take the number in front of the 5 – in this case it is 3. o Add one to the number – in this example that would be 1 + 3 = 4 o Take this number and multiple by the original number; here we get 4 x 3 = 12. o Take this number and put 25 at the end. Here we would get 1225. ...
... o Take the number in front of the 5 – in this case it is 3. o Add one to the number – in this example that would be 1 + 3 = 4 o Take this number and multiple by the original number; here we get 4 x 3 = 12. o Take this number and put 25 at the end. Here we would get 1225. ...
Aalborg Universitet Spontaneous emission in two-dimensional photonic crystal microcavities Søndergaard, Thomas
... states must be compared to a proper reference. For the structures considered in this paper, where the active medium is assumed to be placed in the high-index material, a proper reference is the photon density of states in a homogeneous dielectric with the same dielectric constant as the high-index m ...
... states must be compared to a proper reference. For the structures considered in this paper, where the active medium is assumed to be placed in the high-index material, a proper reference is the photon density of states in a homogeneous dielectric with the same dielectric constant as the high-index m ...
Discussion Questions
... a. polar solute versus nonpolar solute b. KF versus C6H12O6 c. RbCl versus AgCl d. HNO3 versus CO 16. Commercial cold packs and hot packs are available for treating athletic injuries. Both types contain a pouch of water and a dry chemical. When the pack is struck, the pouch of water break ...
... a. polar solute versus nonpolar solute b. KF versus C6H12O6 c. RbCl versus AgCl d. HNO3 versus CO 16. Commercial cold packs and hot packs are available for treating athletic injuries. Both types contain a pouch of water and a dry chemical. When the pack is struck, the pouch of water break ...
answer ch6 - Mr Khaled Nasr
... (9) The weight of a substance in 100 grams of its solution. (10)The number of moles of solute per liter of solution. (11)A solution containing 1 mole of solute in 1000mL of solution. (12)A method of quantitative analysis that is based on measurement of the volume of the substance to be analyzed. (13 ...
... (9) The weight of a substance in 100 grams of its solution. (10)The number of moles of solute per liter of solution. (11)A solution containing 1 mole of solute in 1000mL of solution. (12)A method of quantitative analysis that is based on measurement of the volume of the substance to be analyzed. (13 ...
Crystallization

Crystallization is the (natural or artificial) process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction.