Chapter 11 Chemical Reactions
... There are probably millions of reactions. We can’t remember them all, but luckily they will fall into several categories. We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. How? We recogni ...
... There are probably millions of reactions. We can’t remember them all, but luckily they will fall into several categories. We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. How? We recogni ...
ELECTROLYTES AND NONELECTROLYTES Lec.3
... sugar molecules, surrounded by water molecules, are neutral. When a pair of electrodes is placed in this solution, the sugar molecules are not attracted by either electrode. Consequently no electric current flows through the solution. ☼ Acids are substances that dissociate in water to yield electric ...
... sugar molecules, surrounded by water molecules, are neutral. When a pair of electrodes is placed in this solution, the sugar molecules are not attracted by either electrode. Consequently no electric current flows through the solution. ☼ Acids are substances that dissociate in water to yield electric ...
Vocabulary CHEM121
... Polonium When these are the negative parts of 2-element (binary) compounds, they have an oxidation number of -2. ...
... Polonium When these are the negative parts of 2-element (binary) compounds, they have an oxidation number of -2. ...
PPt3 - WordPress.com
... • The potential is applied between the reference electrode and the working electrode and the current is measured between the working electrode and the counter electrode. ...
... • The potential is applied between the reference electrode and the working electrode and the current is measured between the working electrode and the counter electrode. ...
document
... When compounds containing polyatomic ions dissociate, the polyatomic group stays together as one ion. ...
... When compounds containing polyatomic ions dissociate, the polyatomic group stays together as one ion. ...
Chemical equilibrium, redox and pE
... • Entropy is the degree of disorder of the system chance of finding something in a fixed volume- higher pressure, greater chance, liquid lower entropy than gas, solid lower than liquid • Change in entropy measures capacity for spontaneous change diffusion of a solute from high concentration to low ...
... • Entropy is the degree of disorder of the system chance of finding something in a fixed volume- higher pressure, greater chance, liquid lower entropy than gas, solid lower than liquid • Change in entropy measures capacity for spontaneous change diffusion of a solute from high concentration to low ...
Review Session Handout from 10/6
... 14. Oxalic acid, H2C2O4, is a toxic substance found in spinach leaves, what is the molarity of a solution made by dissolving 12.0 g of oxalic acid in enough water to give 400.0 mL of solution? How many mL of 0.100 M KOH would you need to titrate 25.0 mL of the oxalic acid solution according to the f ...
... 14. Oxalic acid, H2C2O4, is a toxic substance found in spinach leaves, what is the molarity of a solution made by dissolving 12.0 g of oxalic acid in enough water to give 400.0 mL of solution? How many mL of 0.100 M KOH would you need to titrate 25.0 mL of the oxalic acid solution according to the f ...
Final Review
... 8. From your knowledge of intermolecular forces, arrange the following in order of increasing surface tension (least to most): Water, hexane, ethanol, ethanal 9. Describe how the intermolecular forces in water allow for each of the following properties of water: a. low vapor pressure c. solid H2O is ...
... 8. From your knowledge of intermolecular forces, arrange the following in order of increasing surface tension (least to most): Water, hexane, ethanol, ethanal 9. Describe how the intermolecular forces in water allow for each of the following properties of water: a. low vapor pressure c. solid H2O is ...
UNIT 1 - MATTER AND CHEMICAL BONDING
... c) calcium carbonate calcium oxide + carbon dioxide d) propane + oxygen carbon dioxide + water e) lead(II) hydroxide lead(II) oxide + water f) ammonia + sulphuric acid ammonium sulphate g) potassium phosphate + magnesium chloride magnesium phosphate + potassium chloride 6. For each of the ...
... c) calcium carbonate calcium oxide + carbon dioxide d) propane + oxygen carbon dioxide + water e) lead(II) hydroxide lead(II) oxide + water f) ammonia + sulphuric acid ammonium sulphate g) potassium phosphate + magnesium chloride magnesium phosphate + potassium chloride 6. For each of the ...
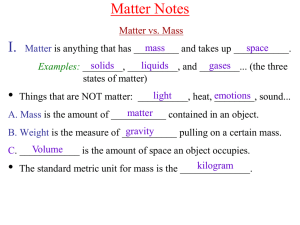
Chem A Week 2 Matter Notes
... chromatography. It is the physical separation of a mixture into its individual components. It involves using a solvent to pass through the mixture. What solvent should I use? The solvent used depends upon the solubility of the mixture you are trying to separate. Examples are water, isopropyl alcohol ...
... chromatography. It is the physical separation of a mixture into its individual components. It involves using a solvent to pass through the mixture. What solvent should I use? The solvent used depends upon the solubility of the mixture you are trying to separate. Examples are water, isopropyl alcohol ...
Lecture 1
... processes, daily variation Climate / climatology – seasonal average of weather at some location Climate change – long term variation in climate ...
... processes, daily variation Climate / climatology – seasonal average of weather at some location Climate change – long term variation in climate ...
Chapters 14
... 7. What is the molarity of a solution made by dissolving 9.1 g of H3PO4 in enough water to make 22.3 L of solution? Assume that H3PO4 ionizes completely in water to H+ and PO43ions. What is the pH of the solution? Find the concentration of OH-? ...
... 7. What is the molarity of a solution made by dissolving 9.1 g of H3PO4 in enough water to make 22.3 L of solution? Assume that H3PO4 ionizes completely in water to H+ and PO43ions. What is the pH of the solution? Find the concentration of OH-? ...
Chemistry PowerPoint
... a. The total mass of the reactants is greater than the total mass of the products b. The total mass of the reactants is less than the total mass of the products c. The total mass of the reactants equals the total mass of the products d. Mass can be created and destroyed ...
... a. The total mass of the reactants is greater than the total mass of the products b. The total mass of the reactants is less than the total mass of the products c. The total mass of the reactants equals the total mass of the products d. Mass can be created and destroyed ...
Document
... parameters include changes in free energy (G), enthalpy (H), entropy (S), volume (V), and heat capacity (Cp) - The laws of thermodynamics provide general constraints that such systems must not violate—of the four laws of thermodynamics (zeroth through third), the first and second laws are of pa ...
... parameters include changes in free energy (G), enthalpy (H), entropy (S), volume (V), and heat capacity (Cp) - The laws of thermodynamics provide general constraints that such systems must not violate—of the four laws of thermodynamics (zeroth through third), the first and second laws are of pa ...
sample paper chemistry clas xi set 3
... (b) Ionization enthalpy of nitrogen is greater than that of oxygen. (c) Electron gain enthalpies of beryllium and magnesium are positive. ...
... (b) Ionization enthalpy of nitrogen is greater than that of oxygen. (c) Electron gain enthalpies of beryllium and magnesium are positive. ...
Sample Paper - Army Public School Jammu Cantt
... 3. Amongst the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields a single monochloride. 4. Give the IUPAC name and structure of the amine obtained when 3-chlorobutanamide undergoes Hoffmann –bromamide reaction. ...
... 3. Amongst the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields a single monochloride. 4. Give the IUPAC name and structure of the amine obtained when 3-chlorobutanamide undergoes Hoffmann –bromamide reaction. ...
AP Chemistry Summer Assignment
... 32. Solid lithium hydroxide is used in space vehicles to remove exhaled carbon dioxide from the living environment by forming solid lithium carbonate and liquid water. What mass of gaseous carbon dioxide can be absorbed by 1.00 x 103 g of lithium hydroxide? ...
... 32. Solid lithium hydroxide is used in space vehicles to remove exhaled carbon dioxide from the living environment by forming solid lithium carbonate and liquid water. What mass of gaseous carbon dioxide can be absorbed by 1.00 x 103 g of lithium hydroxide? ...
Atomic Structure
... Review for 3rd Quarterly Examination - Atomic Structure, Solutions, Kinetics & Equilibrium ...
... Review for 3rd Quarterly Examination - Atomic Structure, Solutions, Kinetics & Equilibrium ...
Mass-Mass Stoichiometry
... b) If in another experiment involving the same reaction 0.75 moles of MgO is produced, how many moles of Oxygen gas must have been reacted? Mass-Mass Stoichiometry 2. Given the following reaction….. Al + Fe2O3 --- Fe + Al2O3 a) If we start with 2.50 grams of Aluminum and an excess of Iron (III) Oxi ...
... b) If in another experiment involving the same reaction 0.75 moles of MgO is produced, how many moles of Oxygen gas must have been reacted? Mass-Mass Stoichiometry 2. Given the following reaction….. Al + Fe2O3 --- Fe + Al2O3 a) If we start with 2.50 grams of Aluminum and an excess of Iron (III) Oxi ...
Electrolysis answers - Barnard Castle School
... (if they give no units or g this is acceptable but other units -1 mark) (if they do not get the correct answer we then look for working marks general principle is one mark lost for each mistake) giving the equation volume / 24000 = mass of gas / Mr (one mark) answer 0.0355 = 2 marks (without working ...
... (if they give no units or g this is acceptable but other units -1 mark) (if they do not get the correct answer we then look for working marks general principle is one mark lost for each mistake) giving the equation volume / 24000 = mass of gas / Mr (one mark) answer 0.0355 = 2 marks (without working ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.