7th Chemistry Unit Test Study Guide Test Date: Friday, Nov. 16
... What does the Law of Conservation of Mass state? ...
... What does the Law of Conservation of Mass state? ...
Introductory Chemistry Test Review
... 25. In the laboratory, potassium chlorate will decompose when heated to form potassium chloride and oxygen gas according to the following equation. Calculate how much oxygen in grams is produced when 35.0 grams of potassium chlorate decomposes. 2 KClO3(s) ...
... 25. In the laboratory, potassium chlorate will decompose when heated to form potassium chloride and oxygen gas according to the following equation. Calculate how much oxygen in grams is produced when 35.0 grams of potassium chlorate decomposes. 2 KClO3(s) ...
document
... ___T______20. The number of atoms on both sides of an equation must be equal for each element. Part C: For each of the following compounds, identify the number of atoms of each element. ...
... ___T______20. The number of atoms on both sides of an equation must be equal for each element. Part C: For each of the following compounds, identify the number of atoms of each element. ...
The bombardier beetle uses an explosive discharge as a defensive
... 2. A hot air balloon is being inflated to its full extent by heating the air inside it. In the final stages of this process, the volume of the balloon changes from 3.5 x 106 L to 4.50 x 106 L by the addition of 160 MJ of energy as heat. Assuming that the balloon expands against a constant pressure o ...
... 2. A hot air balloon is being inflated to its full extent by heating the air inside it. In the final stages of this process, the volume of the balloon changes from 3.5 x 106 L to 4.50 x 106 L by the addition of 160 MJ of energy as heat. Assuming that the balloon expands against a constant pressure o ...
Chemistry Unit Review
... b. When sugar (C12H22O11) and sulfuric acid (H2SO4) are combined, carbon, water, and sulfur dioxide are formed. ...
... b. When sugar (C12H22O11) and sulfuric acid (H2SO4) are combined, carbon, water, and sulfur dioxide are formed. ...
Chemical Equations TrackStar Assignment
... 4. Write the reaction for the burning of Methane gas (the gas used in Chemistry lab). What type of reaction is this? 5. Write the reaction of the neutralization of stomach acid. What type of reaction is this? 6. Does the order in which the reactants and products are written in the chemical equation ...
... 4. Write the reaction for the burning of Methane gas (the gas used in Chemistry lab). What type of reaction is this? 5. Write the reaction of the neutralization of stomach acid. What type of reaction is this? 6. Does the order in which the reactants and products are written in the chemical equation ...
Ch. 1-- Matter and Change
... with each other are written on the _______ left and are called “reactants”. The substances that are ____________ produced are written on the _______ right and are called the “products.” Reactants Products ...
... with each other are written on the _______ left and are called “reactants”. The substances that are ____________ produced are written on the _______ right and are called the “products.” Reactants Products ...
students - Teach-n-Learn-Chem
... -- “soluble” or “in solution” also indicate that a substance is dissolved in water (usually) -- acids are aqueous solutions Other symbols… means “yields” or “produces” means heat is added to the reaction ...
... -- “soluble” or “in solution” also indicate that a substance is dissolved in water (usually) -- acids are aqueous solutions Other symbols… means “yields” or “produces” means heat is added to the reaction ...
+ H 2 SO 4(aq) - Rothschild Science
... different molecules switch places, forming two entirely different compounds ...
... different molecules switch places, forming two entirely different compounds ...
Chemical Equations and Reaction Types Lab
... molecular equations and as ionic equations. We shall only consider molecular equations in this exercise. ...
... molecular equations and as ionic equations. We shall only consider molecular equations in this exercise. ...
Chemical Equations and Reactions
... Hg (mercury) can exist by itself...but, oxygen will need to bond with another oxygen to make O2 (diatomic) To balance the atoms we need to: Put the coefficient of 2 in front of reactant HgO. Put the coefficient of 2 in front the product Hg. ...
... Hg (mercury) can exist by itself...but, oxygen will need to bond with another oxygen to make O2 (diatomic) To balance the atoms we need to: Put the coefficient of 2 in front of reactant HgO. Put the coefficient of 2 in front the product Hg. ...
Dr. Audrey Lugo`s AP Chemistry Course Syllabus
... one day late on chapter problems or chapter notes, up to two days late. 2 Mole Bucks equal one day late on major projects (such as lab reports). Any remaining Mole Bucks at the end of the semester may be used as one point on the final exam. After two days with Mole Bucks or on the first day late wit ...
... one day late on chapter problems or chapter notes, up to two days late. 2 Mole Bucks equal one day late on major projects (such as lab reports). Any remaining Mole Bucks at the end of the semester may be used as one point on the final exam. After two days with Mole Bucks or on the first day late wit ...
Unit 13 - Electrochemistry
... the reducing agent. - The substance that is reduced is called the oxidizing agent. - Single replacement and combustion reactions are redox reactions, double replacement is not a redox reaction. ...
... the reducing agent. - The substance that is reduced is called the oxidizing agent. - Single replacement and combustion reactions are redox reactions, double replacement is not a redox reaction. ...
Toluenediamine
... enhanced selectivity. Since recent developments the promoters have overcome the common alloying elements (Fe, Cr, Cu and Mo) and rather comprise the entire periodic table. Not only the chemical additives can influence the catalyst performance after leaching but also the alloy manufacturing process. ...
... enhanced selectivity. Since recent developments the promoters have overcome the common alloying elements (Fe, Cr, Cu and Mo) and rather comprise the entire periodic table. Not only the chemical additives can influence the catalyst performance after leaching but also the alloy manufacturing process. ...
Chapter 2 Chemical Reactions
... Now, read these equations: Fe(s) + O2(g) Fe2O3(s) Cu(s) + AgNO3(aq) Ag(s) + Cu(NO3)2(aq) Pt ...
... Now, read these equations: Fe(s) + O2(g) Fe2O3(s) Cu(s) + AgNO3(aq) Ag(s) + Cu(NO3)2(aq) Pt ...
Free Energy I
... If only P-V work is performed, the change in Gibbs Free Energy ΔG is the maximum work* that can be obtained from a chemical reaction at constant temperature ...
... If only P-V work is performed, the change in Gibbs Free Energy ΔG is the maximum work* that can be obtained from a chemical reaction at constant temperature ...
Chemistry Midterm Review Sheet
... a) Write the balanced equation for the reaction b) How many moles of water were formed? How many molecules? c) How many moles of butane burned? d) How many grams of butane burned? e) How many grams and moles of oxygen gas were used up? f) What is the mass percentage of carbon in butane? In the late ...
... a) Write the balanced equation for the reaction b) How many moles of water were formed? How many molecules? c) How many moles of butane burned? d) How many grams of butane burned? e) How many grams and moles of oxygen gas were used up? f) What is the mass percentage of carbon in butane? In the late ...
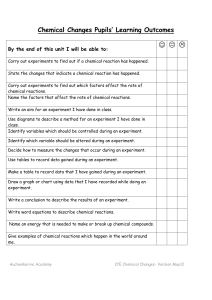
Chemical Reactions Unit Pupils` Learning Outcomes
... Chemical Changes Pupils’ Learning Outcomes ...
... Chemical Changes Pupils’ Learning Outcomes ...
groups (families) vs rows
... How many grams of SnF2 are produced from the reaction of 30.00g of HF with Sn? LIMITING REACTANT PROBLEMS ...
... How many grams of SnF2 are produced from the reaction of 30.00g of HF with Sn? LIMITING REACTANT PROBLEMS ...
Chapter 18 Review 18.1 Oxidation-Reduction Reactions Oxidation
... Electrochemistry- a study of the interchange of chemical and electrical energy Galvanic Cell (aka electrochemical battery)- a device powered by an redox reaction where the agents are separated, the electrons flow through a wire, and there is a salt bridge connecting the two solutions Anode- the elec ...
... Electrochemistry- a study of the interchange of chemical and electrical energy Galvanic Cell (aka electrochemical battery)- a device powered by an redox reaction where the agents are separated, the electrons flow through a wire, and there is a salt bridge connecting the two solutions Anode- the elec ...