study guide and review for first semester final
... and net ionic equations for the reaction. H2SO4 + 2 KOH K2SO4 + 2 H2O Yes because water is a product Net Ionic 2H+(aq) + 2OH-(aq) 2H2O(l) 18. Work stoichiometric problems using ionic equations. Ex. How many mL of 0.100 M AgNO3 solution are needed to react completely with 25.0 mL of 0.400 M CaCl2 ...
... and net ionic equations for the reaction. H2SO4 + 2 KOH K2SO4 + 2 H2O Yes because water is a product Net Ionic 2H+(aq) + 2OH-(aq) 2H2O(l) 18. Work stoichiometric problems using ionic equations. Ex. How many mL of 0.100 M AgNO3 solution are needed to react completely with 25.0 mL of 0.400 M CaCl2 ...
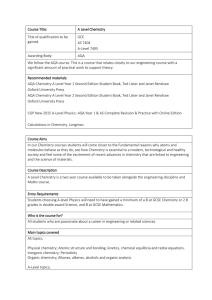
A-level Chemistry
... Teaching and learning methods used Teaching and learning methods used include lectures, group work, extensive practical work, independent learning and external workshops through professional membership of external organisations How your work will be assessed Routine formative and summative assessmen ...
... Teaching and learning methods used Teaching and learning methods used include lectures, group work, extensive practical work, independent learning and external workshops through professional membership of external organisations How your work will be assessed Routine formative and summative assessmen ...
Chapter 1
... Stoichiometric factors (or molar ratios) may be used to convert between quantities of reactants and products in a reaction. It is important to realize that the stoichiometric ratios are the ideal proportions in which reactants are needed to form products. A balanced reaction equation often provides ...
... Stoichiometric factors (or molar ratios) may be used to convert between quantities of reactants and products in a reaction. It is important to realize that the stoichiometric ratios are the ideal proportions in which reactants are needed to form products. A balanced reaction equation often provides ...
Document
... • Aluminum burns in bromine producing aluminum bromide. In a laboratory 6.0 g of aluminum reacts with excess bromine. 50.3 g of aluminum bromide are produced. What are the three types of yield? ...
... • Aluminum burns in bromine producing aluminum bromide. In a laboratory 6.0 g of aluminum reacts with excess bromine. 50.3 g of aluminum bromide are produced. What are the three types of yield? ...
Partial Pressures of Gases
... Steps for Balancing Chemical Equations by Inspection 1. Write the chemical formula for each reactant and product, including the state of matter for each one. 2. Try balancing any atom that is not in a polyatomic ion and is not carbon, oxygen or hydrogen.(ECHO) 3. If possible, balance polyatomic ions ...
... Steps for Balancing Chemical Equations by Inspection 1. Write the chemical formula for each reactant and product, including the state of matter for each one. 2. Try balancing any atom that is not in a polyatomic ion and is not carbon, oxygen or hydrogen.(ECHO) 3. If possible, balance polyatomic ions ...
percent composition and formulas
... • In an organic chemistry lab, 12.4 grams of an organic compound was produced. According to calculations, the reaction should have produced 17.0 grams. Calculate the percent yield. ...
... • In an organic chemistry lab, 12.4 grams of an organic compound was produced. According to calculations, the reaction should have produced 17.0 grams. Calculate the percent yield. ...
Predicting Products online assistance #3
... 1. synthesis - two reactants combine to form one product 2. decomposition - one reactant decomposes, or breaks apart, into two or more products. 3. single replacement - an element replaces another in a compound. 4. double replacement - the elements in two compounds switch partners to form two new co ...
... 1. synthesis - two reactants combine to form one product 2. decomposition - one reactant decomposes, or breaks apart, into two or more products. 3. single replacement - an element replaces another in a compound. 4. double replacement - the elements in two compounds switch partners to form two new co ...
Lecture syllabus - Linfield College
... explain it verbally to someone else in the course. If your listener grasps the concept easily from your explanation, you have proven that you understand it. If not, then most likely you need to work on clarifying your own grasp of it. It is also easy to discover whether or not you know a reaction or ...
... explain it verbally to someone else in the course. If your listener grasps the concept easily from your explanation, you have proven that you understand it. If not, then most likely you need to work on clarifying your own grasp of it. It is also easy to discover whether or not you know a reaction or ...
Types of Chemical Reactions
... List three types of synthesis reactions and six types of decomposition reactions. List four types of single-replacement reactions and three types of doublereplacement reactions. Predict the products of single reactions given the reactants. ...
... List three types of synthesis reactions and six types of decomposition reactions. List four types of single-replacement reactions and three types of doublereplacement reactions. Predict the products of single reactions given the reactants. ...
chem 111 practice exam
... (15 points) 8. Hydrogen cyanide is prepared from ammonia, air, and natural gas, CH4 by the : 2 NH3 + 3 O2 +2CH4---> 2HCN + 6 H2O If a reaction vessel contains 1.15 g NH3, 1.00 g oxygen, and 1.05 g CH4 produces 0 .400 g HCN, what is the percent ...
... (15 points) 8. Hydrogen cyanide is prepared from ammonia, air, and natural gas, CH4 by the : 2 NH3 + 3 O2 +2CH4---> 2HCN + 6 H2O If a reaction vessel contains 1.15 g NH3, 1.00 g oxygen, and 1.05 g CH4 produces 0 .400 g HCN, what is the percent ...
Unit 3 - sotochem
... the lowest whole number ratio of atoms in a chemical formula ○ From % composition, empirical formula can be determined using mole ratios ○ Percent to mass, mass to mole, divide by small, multiply till whole ■ Use the percentages as mass measurements out of 100 g. Convert these masses to mole amounts ...
... the lowest whole number ratio of atoms in a chemical formula ○ From % composition, empirical formula can be determined using mole ratios ○ Percent to mass, mass to mole, divide by small, multiply till whole ■ Use the percentages as mass measurements out of 100 g. Convert these masses to mole amounts ...
Chapter 3
... convert moles of one reactant to moles of other reactants and products (use the stoichiometric ratio from the balanced chemical equation), and then ...
... convert moles of one reactant to moles of other reactants and products (use the stoichiometric ratio from the balanced chemical equation), and then ...
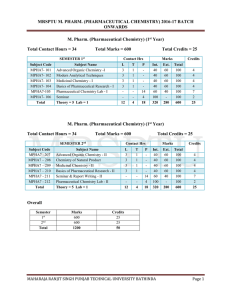
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
Solution
... favored. This is consistent with the negative free energy of part (c). e) The pressure of oxygen is 5 atm and the pressure of hydrogen is 10 atm at 25°C. In which direction will the reaction shift in order to regain equilibrium. Show all work and explain your reasoning. We have K calculated above an ...
... favored. This is consistent with the negative free energy of part (c). e) The pressure of oxygen is 5 atm and the pressure of hydrogen is 10 atm at 25°C. In which direction will the reaction shift in order to regain equilibrium. Show all work and explain your reasoning. We have K calculated above an ...
Thermochemistry: The Heat of Neutralization
... enthalpy value (∆H < 0). Most reactions occur in several steps, with energy required (∆H > 0) to break bonds, and energy released (∆H < 0) as new bonds are formed. If a reaction can be written as the sum of several individual reactions, the enthalpies of the individual reactions will add up to give ...
... enthalpy value (∆H < 0). Most reactions occur in several steps, with energy required (∆H > 0) to break bonds, and energy released (∆H < 0) as new bonds are formed. If a reaction can be written as the sum of several individual reactions, the enthalpies of the individual reactions will add up to give ...
MOLECULAR FORMULAS N C H H C N H HHH HH
... 4. (9 points) Gold, Au, is dissolved from rock by treating the rock with NaCN in the presence of oxygen. 4 Au(s) + 8 NaCN(aq) + O2(g) + 2 H2O(l) → 4 NaAu(CN)2(aq) + 4 NaOH(aq) (a) If you have 0.050 mol of gold, the number of moles of NaCN required is __________ mol and the number of moles of O2 requ ...
... 4. (9 points) Gold, Au, is dissolved from rock by treating the rock with NaCN in the presence of oxygen. 4 Au(s) + 8 NaCN(aq) + O2(g) + 2 H2O(l) → 4 NaAu(CN)2(aq) + 4 NaOH(aq) (a) If you have 0.050 mol of gold, the number of moles of NaCN required is __________ mol and the number of moles of O2 requ ...
Document
... Aluminum burns in bromine producing aluminum bromide. In a laboratory 6.0 g of aluminum reacts with excess bromine. 50.3 g of aluminum bromide are produced. What are the three types of yield. ...
... Aluminum burns in bromine producing aluminum bromide. In a laboratory 6.0 g of aluminum reacts with excess bromine. 50.3 g of aluminum bromide are produced. What are the three types of yield. ...
Chemical Reactions Chemical Arithmetic
... reaction in which the oxidation numbers of elements change because of a loss or gain of electrons • Oxidation Number- A number that indicates the charge that an atom in a molecule or polyatomic ion would have if all bonds were ionic. – Fictitious- No actual charge of this magnitude actually exists w ...
... reaction in which the oxidation numbers of elements change because of a loss or gain of electrons • Oxidation Number- A number that indicates the charge that an atom in a molecule or polyatomic ion would have if all bonds were ionic. – Fictitious- No actual charge of this magnitude actually exists w ...
physics/0010052 PDF
... 0,03%, respectively. The equilibrium constant at 273 K deflects from this relationship. Dependence of K on temperature is a broken line. The points 293, 303 and 323 K are at one segment, 273 K is at the other one. From Eqs. (7) and (8) it is possible to calculate ∆H*0 and ∆Q0=∆H*0- P∆V0. We suppose ...
... 0,03%, respectively. The equilibrium constant at 273 K deflects from this relationship. Dependence of K on temperature is a broken line. The points 293, 303 and 323 K are at one segment, 273 K is at the other one. From Eqs. (7) and (8) it is possible to calculate ∆H*0 and ∆Q0=∆H*0- P∆V0. We suppose ...
Chapter 3. Stoichiometry: Calculations with Chemical Formulas and
... 3.7 Limiting Reactants • Not necessary to have all reactants present in stoichiometric amounts • Often, one or more reactants is present in excess • At the end of reaction those reactants present in excess will still be in the reaction mixture • The one or more reactants completely consumed are cal ...
... 3.7 Limiting Reactants • Not necessary to have all reactants present in stoichiometric amounts • Often, one or more reactants is present in excess • At the end of reaction those reactants present in excess will still be in the reaction mixture • The one or more reactants completely consumed are cal ...