Chapter 12 Packet
... iii. If 67L of nitrogen gas were produced upon impact, would 50g of iron (III) oxide be sufficient to convert all of the sodium to sodium oxide. Hint: first determine what mass of sodium would be produced in reaction (2a) and then determine the limiting reactant in (2ci). ...
... iii. If 67L of nitrogen gas were produced upon impact, would 50g of iron (III) oxide be sufficient to convert all of the sodium to sodium oxide. Hint: first determine what mass of sodium would be produced in reaction (2a) and then determine the limiting reactant in (2ci). ...
Theoretical Calculation of Enthalpy of reactions involved in PZ
... Heat of absorption is a very important thermodynamic parameter in CO2 removal using amine solvent solutions in post combustion temperature swing processes. The temperature dependency of the heat of absorption for such processes can be calculated from the theoretical equilibrium constants [1] for eac ...
... Heat of absorption is a very important thermodynamic parameter in CO2 removal using amine solvent solutions in post combustion temperature swing processes. The temperature dependency of the heat of absorption for such processes can be calculated from the theoretical equilibrium constants [1] for eac ...
Chapter 3. Stoichiometry
... in the laboratory versus the number of moles required by stoichiometry. • Students do not appreciate that the coefficients in an empirical formula are not exact whole numbers because of experimental or round-off errors. In general, students have problems with the existence of experimental error. • T ...
... in the laboratory versus the number of moles required by stoichiometry. • Students do not appreciate that the coefficients in an empirical formula are not exact whole numbers because of experimental or round-off errors. In general, students have problems with the existence of experimental error. • T ...
Chemical Equations
... formulas for the reactants (on the left of the arrow) and the products (on the right of the arrow). C. The law of conservation of mass and energy must be satisfied. Therefore the same number of atoms of each element must appear on each side of a correct chemical equation. ...
... formulas for the reactants (on the left of the arrow) and the products (on the right of the arrow). C. The law of conservation of mass and energy must be satisfied. Therefore the same number of atoms of each element must appear on each side of a correct chemical equation. ...
Chemical Reactions PPT
... • Conservation of atoms-the number of each type of atom on the reactants side of the chemical equation MUST be equal to the number of each type of atom on the products side of the equation. • Coefficient-represent the number of units of each substance taking part in the reaction ...
... • Conservation of atoms-the number of each type of atom on the reactants side of the chemical equation MUST be equal to the number of each type of atom on the products side of the equation. • Coefficient-represent the number of units of each substance taking part in the reaction ...
CP Chemistry Midterm Study Guide
... Use the following information for questions 37 & 38: Ammonium nitrate (NH4NO3) is an important fertilizer and is also used in the manufacture of explosives and fireworks. It is produced by treating nitric acid (HNO3) with ammonia gas (NH3). The balanced equation for this reaction is: HNO3 + NH3 NH ...
... Use the following information for questions 37 & 38: Ammonium nitrate (NH4NO3) is an important fertilizer and is also used in the manufacture of explosives and fireworks. It is produced by treating nitric acid (HNO3) with ammonia gas (NH3). The balanced equation for this reaction is: HNO3 + NH3 NH ...
Put Paper Title Here
... accelerator-driven sub-critical reactor for transmutation. Other schemes under consideration involve intermediate critical reactor steps; this would result in major changes in the design, development and analysis of separations systems. Systems engineering would enhance the ability to respond with s ...
... accelerator-driven sub-critical reactor for transmutation. Other schemes under consideration involve intermediate critical reactor steps; this would result in major changes in the design, development and analysis of separations systems. Systems engineering would enhance the ability to respond with s ...
Wk-11-14
... C and D. Problem is: we may not necessarily agree! Western cultures (and chemists of all cultures) try to manipulate equilibrium, as if it is our manifest destiny to do so! ...
... C and D. Problem is: we may not necessarily agree! Western cultures (and chemists of all cultures) try to manipulate equilibrium, as if it is our manifest destiny to do so! ...
Mock Final Exam
... from 23 to 30.5. The specific heat of aluminum is 0.90 J/g-K. What is the mass of the aluminum sample? 82. 50.0 mL of 1.0 M HCl were mixed with 50.0 mL of 1.0 M NaOH in a ‘coffee cup’ calorimeter. The solution warmed from 23.0 to 29.8. Assume the solution had the density and specific heat of pur ...
... from 23 to 30.5. The specific heat of aluminum is 0.90 J/g-K. What is the mass of the aluminum sample? 82. 50.0 mL of 1.0 M HCl were mixed with 50.0 mL of 1.0 M NaOH in a ‘coffee cup’ calorimeter. The solution warmed from 23.0 to 29.8. Assume the solution had the density and specific heat of pur ...
Lecture 5 – Chemical Reactions
... Rule 6: The algebraic sum of the O.N.’s of all atoms in a complete compound formula equals zero. g. Rule 7: The algebraic sum of the O.N.’s of all atoms in a poly atomic ion is equal to the charge on the ion. If in a reaction the oxidation number for an element increases, it is oxidized; conversely, ...
... Rule 6: The algebraic sum of the O.N.’s of all atoms in a complete compound formula equals zero. g. Rule 7: The algebraic sum of the O.N.’s of all atoms in a poly atomic ion is equal to the charge on the ion. If in a reaction the oxidation number for an element increases, it is oxidized; conversely, ...
Chemistry in Society - Cathkin High School
... distillation. Naphtha is a feedstock that can be cracked to produce ethene. Batch and Continuous Processes In a batch process the chemicals are loaded into the reaction vessel. The reaction is monitored and at the end of the reaction the product is separated and the reaction vessel cleaned out ready ...
... distillation. Naphtha is a feedstock that can be cracked to produce ethene. Batch and Continuous Processes In a batch process the chemicals are loaded into the reaction vessel. The reaction is monitored and at the end of the reaction the product is separated and the reaction vessel cleaned out ready ...
Section 2 Types of Chemical Reactions Chapter 8
... FeS(s) + 2HCl(aq) H2S(g) + FeCl2(aq) HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) ...
... FeS(s) + 2HCl(aq) H2S(g) + FeCl2(aq) HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) ...
Chapter 7
... resulting from a collision of atoms or molecules. • The original substances are reactants • The substances produced by the reaction are called products for example: carbon can collide with oxygen and make carbon dioxide Chemical Equation: ...
... resulting from a collision of atoms or molecules. • The original substances are reactants • The substances produced by the reaction are called products for example: carbon can collide with oxygen and make carbon dioxide Chemical Equation: ...
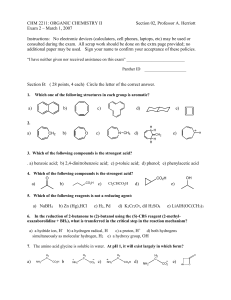
Exam 2
... Org II exam, p.2 Section A (48 points, 4 each – answer 12 of the 13 questions, marking clearly the one you choose to omit; if none is marked, the last answer will be omitted) Give the major organic product(s) of the following reactions, including stereochemistry if appropriate. ...
... Org II exam, p.2 Section A (48 points, 4 each – answer 12 of the 13 questions, marking clearly the one you choose to omit; if none is marked, the last answer will be omitted) Give the major organic product(s) of the following reactions, including stereochemistry if appropriate. ...
Export To Word
... Standard: Matter A. A working definition of matter is that it takes up space, has mass, and has measurable properties. Matter is comprised of atomic, subatomic, and elementary particles. B. Electrons are key to defining chemical and some physical properties, reactivity, and molecular structures. Rep ...
... Standard: Matter A. A working definition of matter is that it takes up space, has mass, and has measurable properties. Matter is comprised of atomic, subatomic, and elementary particles. B. Electrons are key to defining chemical and some physical properties, reactivity, and molecular structures. Rep ...
2014 Academic Challenge Sectional Chemistry Exam Solution Set 1
... the forward reaction is the vertical distance from the reactants to the top of the hill. The activation energy of the reverse reaction is the distance from the products to the top of the hill. The exothermic nature of the reaction requires EAfwd to be less than EArev. It is not required that this ob ...
... the forward reaction is the vertical distance from the reactants to the top of the hill. The activation energy of the reverse reaction is the distance from the products to the top of the hill. The exothermic nature of the reaction requires EAfwd to be less than EArev. It is not required that this ob ...
Regents Exam In Chemistry Review Homework #1
... 7) What happens to the boiling point of water if a solute is dissolved into it?__________________________________ ...
... 7) What happens to the boiling point of water if a solute is dissolved into it?__________________________________ ...
ppt
... Sample Problem 1 From a calorimetry experiment, a student determines that the specific heat of solution of KBrO3 (s) is an endothermic 0.25 kJ/g. Calculate the molar heat of solution and write the thermochemical equation. ...
... Sample Problem 1 From a calorimetry experiment, a student determines that the specific heat of solution of KBrO3 (s) is an endothermic 0.25 kJ/g. Calculate the molar heat of solution and write the thermochemical equation. ...
Chapter 3 Part 2 Review
... Do now: Nitrogen monoxide gas reacts with oxygen gas to produce nitrogen dioxide gas. If 5.00 grams of nitrogen monoxide reacts with excess oxygen, how many grams of nitrogen dioxide gas are ...
... Do now: Nitrogen monoxide gas reacts with oxygen gas to produce nitrogen dioxide gas. If 5.00 grams of nitrogen monoxide reacts with excess oxygen, how many grams of nitrogen dioxide gas are ...
Chapter 12 - "Chemical Formulas and Equations"
... – An oxidation reduction reaction is one in which electrons are transferred between atoms. – Oxidation is the loss of electrons – Reduction is the gain of electrons – Oxidizing agents are substances which take electrons away from other atoms. • An oxidizing agent is reduced when it oxidizes another ...
... – An oxidation reduction reaction is one in which electrons are transferred between atoms. – Oxidation is the loss of electrons – Reduction is the gain of electrons – Oxidizing agents are substances which take electrons away from other atoms. • An oxidizing agent is reduced when it oxidizes another ...
AP Chem Summer Assignment
... Welcome to AP Chemistry. You have chosen to take a very challenging class. It is designed to be the equivalent of a first year college general chemistry course. That said, it is intended for motivated and mature high school students who are genuinely interested in chemistry. Since you are expected t ...
... Welcome to AP Chemistry. You have chosen to take a very challenging class. It is designed to be the equivalent of a first year college general chemistry course. That said, it is intended for motivated and mature high school students who are genuinely interested in chemistry. Since you are expected t ...
Notes 2 Balancing
... • The Law of Conservation of Mass • States that in ordinary chemical or physical changes, mass is neither created nor destroyed. • React vinegar and baking soda • Produces a gas (which “floats” away). • The products including this gas, if captured, is the same mass per mole as the reactants consumed ...
... • The Law of Conservation of Mass • States that in ordinary chemical or physical changes, mass is neither created nor destroyed. • React vinegar and baking soda • Produces a gas (which “floats” away). • The products including this gas, if captured, is the same mass per mole as the reactants consumed ...
chemical equation - Central Lyon CSD
... • The heat and smoke of burning charcoal are the products of a combustion reaction. Combustion is one of the five general types of chemical reactions. If you can recognize a reaction as being a particular type, you may be able to predict the products of the reaction. ...
... • The heat and smoke of burning charcoal are the products of a combustion reaction. Combustion is one of the five general types of chemical reactions. If you can recognize a reaction as being a particular type, you may be able to predict the products of the reaction. ...