ch6_f08
... b: Water has a larger specific heat capacity than copper. c: There must be some mistake in these measurements. d: Mass is being converted into energy in this process. ...
... b: Water has a larger specific heat capacity than copper. c: There must be some mistake in these measurements. d: Mass is being converted into energy in this process. ...
1.8 M - Thierry Karsenti
... fundamental concepts and principles associated with chemical reactions notably their rates of reaction,how they are investigated and in paticular, the factors including energy considerations that affect the different rates of reactions.. The module will look at the physical properties of solutions, ...
... fundamental concepts and principles associated with chemical reactions notably their rates of reaction,how they are investigated and in paticular, the factors including energy considerations that affect the different rates of reactions.. The module will look at the physical properties of solutions, ...
Cl 2
... Limiting and Excess Reagents • In a chemical reaction, an insufficient quantity of any of the reactants will limit the amount of product formed. – Limiting reagent is the reactant that determines the amount of product that can be formed by a reaction – Excess reagent is the reactant that is not com ...
... Limiting and Excess Reagents • In a chemical reaction, an insufficient quantity of any of the reactants will limit the amount of product formed. – Limiting reagent is the reactant that determines the amount of product that can be formed by a reaction – Excess reagent is the reactant that is not com ...
Stoichiometry - coercingmolecules
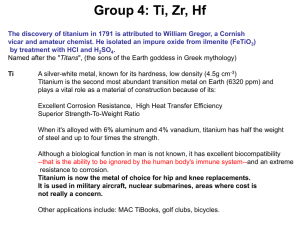
... In one process, 124 g of Al are reacted with 601 g of ferric oxide. (a)Which is the limiting reactant? (b)How much Al2O3 (in grams) is produced? (c)How much of the excess reactant (in grams) is left at the end of the reaction? 2. Titanium is a strong & light metal used in rockets & aircrafts. It is ...
... In one process, 124 g of Al are reacted with 601 g of ferric oxide. (a)Which is the limiting reactant? (b)How much Al2O3 (in grams) is produced? (c)How much of the excess reactant (in grams) is left at the end of the reaction? 2. Titanium is a strong & light metal used in rockets & aircrafts. It is ...
FE Exam Review for Chemistry
... Atoms are the smallest indivisible form of matter that retain the physical & chemical properties of that matter. An element is a type of atom with a defined number of p, n & e‐. What are the three subatomic particles? What do you know about each? Protons = + charge, mass of 1 amu, in the ...
... Atoms are the smallest indivisible form of matter that retain the physical & chemical properties of that matter. An element is a type of atom with a defined number of p, n & e‐. What are the three subatomic particles? What do you know about each? Protons = + charge, mass of 1 amu, in the ...
PHYSICAL SETTING CHEMISTRY
... 58 Determine the total number of electrons in the bonds between the nitrogen atom and the three hydrogen atoms represented in diagram 2. [1] 59 Explain, in terms of distribution of charge, why a molecule of the substance represented in diagram 3 is nonpolar. [1] 60 Draw a Lewis electron-dot diagram ...
... 58 Determine the total number of electrons in the bonds between the nitrogen atom and the three hydrogen atoms represented in diagram 2. [1] 59 Explain, in terms of distribution of charge, why a molecule of the substance represented in diagram 3 is nonpolar. [1] 60 Draw a Lewis electron-dot diagram ...
Reaction of niobium with water
... This should worry you……..how can Cr(VI) be coloured? What is its electron configuration? Colours of the chromate and dichromate ions are the result of charge transfer transitions. Specifically, an electron is transferred from the ligand to the metal. Cr6+-O2- Cr5+-OAn electron in the filled ligand ...
... This should worry you……..how can Cr(VI) be coloured? What is its electron configuration? Colours of the chromate and dichromate ions are the result of charge transfer transitions. Specifically, an electron is transferred from the ligand to the metal. Cr6+-O2- Cr5+-OAn electron in the filled ligand ...
Balanced Equations And Equilibrium Constants
... Given overall reaction (need to REVERSE first reaction, see Rule 2 for how this changes the equilibrium constant): N2O(g) + (1/2)O2(g) <---> 2NO(g) K = (1/K1)*(K2) = 8.5*10-13 2) When we reverse an equation we invert the value of K. Kreverse reaction = 1/(Kforward reaction) 3) When we multiply coeff ...
... Given overall reaction (need to REVERSE first reaction, see Rule 2 for how this changes the equilibrium constant): N2O(g) + (1/2)O2(g) <---> 2NO(g) K = (1/K1)*(K2) = 8.5*10-13 2) When we reverse an equation we invert the value of K. Kreverse reaction = 1/(Kforward reaction) 3) When we multiply coeff ...
Chemical Reactions
... The molar mass of any substance (the mass of one mole of any substance) is the formula weight of the substance expressed in grams. For instance, the formula weight of glucose, C6H12O6 (Example 4.1) is 180 amu; therefore, 180 g of glucose is one mol of glucose. Likewise, the formula weight of urea, ( ...
... The molar mass of any substance (the mass of one mole of any substance) is the formula weight of the substance expressed in grams. For instance, the formula weight of glucose, C6H12O6 (Example 4.1) is 180 amu; therefore, 180 g of glucose is one mol of glucose. Likewise, the formula weight of urea, ( ...
3. What is the empirical formula of a compound that is
... 7.When 10.0 g of copper was reacted with 60.0 g of silver nitrate solution. How many grams of silver are produced? How much of each reactant is left over?( Calculate the amount in grams) ...
... 7.When 10.0 g of copper was reacted with 60.0 g of silver nitrate solution. How many grams of silver are produced? How much of each reactant is left over?( Calculate the amount in grams) ...
Chemistry(I) Final Exam 1/11/2008
... of a to assign each of these two gases to it’s a value. ( (P+an2/V2)(V-nb) = nRT) (3+4 points) (a) M.W. of H2 = 2 , H2O = 18 Î root mean speed of H2 = 640 x (18/2)1/2 = 1920 (m/s) ...
... of a to assign each of these two gases to it’s a value. ( (P+an2/V2)(V-nb) = nRT) (3+4 points) (a) M.W. of H2 = 2 , H2O = 18 Î root mean speed of H2 = 640 x (18/2)1/2 = 1920 (m/s) ...
The masses of reactants and products are equal.
... A balanced chemical equation shows that no matter how atoms are rearranged during a chemical reaction, the same number of atoms must be present before and after the reaction. The following example demonstrates the usefulness of chemical equations and the conservation of mass. The decomposition of so ...
... A balanced chemical equation shows that no matter how atoms are rearranged during a chemical reaction, the same number of atoms must be present before and after the reaction. The following example demonstrates the usefulness of chemical equations and the conservation of mass. The decomposition of so ...
A.P. Chemistry Writing Chemical Reactions Generally students do
... On the actual exam in May you will have 40 minutes (calculator-free) to write three balanced net-ionic reactions and respond to two essay questions. Probably—although it is not yet clear—you will be able to spend whatever part of the 40 minutes you wish on the reactions. You will not be able to choo ...
... On the actual exam in May you will have 40 minutes (calculator-free) to write three balanced net-ionic reactions and respond to two essay questions. Probably—although it is not yet clear—you will be able to spend whatever part of the 40 minutes you wish on the reactions. You will not be able to choo ...
Chapter 3 Atomic Mass
... Cu(s) + AgNO3 (aq) Ag(s) + Cu(NO3)2 (aq) Write the sentence for this reaction: Fe (s) + Cu(NO3)2 (aq) Fe(NO3)2 (aq)+ Cu (s) ...
... Cu(s) + AgNO3 (aq) Ag(s) + Cu(NO3)2 (aq) Write the sentence for this reaction: Fe (s) + Cu(NO3)2 (aq) Fe(NO3)2 (aq)+ Cu (s) ...
CH 4: Chemical Reactions
... • CH3CO2H weak acid doesn’t dissociate well • HCl is a strong acid and therefore a strong electrolyte • NH3 is a weak base and is a weak electrolyte ...
... • CH3CO2H weak acid doesn’t dissociate well • HCl is a strong acid and therefore a strong electrolyte • NH3 is a weak base and is a weak electrolyte ...
CHAPTER 17
... attracted equally in all direction by its neighbors, and there is therefore no resultant force tending to move it in any direction. On the other hand, at the surface of a liquid there is a net attraction of the vapor molecules into the liquid. ...
... attracted equally in all direction by its neighbors, and there is therefore no resultant force tending to move it in any direction. On the other hand, at the surface of a liquid there is a net attraction of the vapor molecules into the liquid. ...
0922085
... 11% (by volume). What would the properties of a mixture of 0.1% (by volume) of aniline and 99.9% (by volume) of air ...
... 11% (by volume). What would the properties of a mixture of 0.1% (by volume) of aniline and 99.9% (by volume) of air ...
Test - Regents
... Setting/Chemistry, and your knowledge of chemistry. In the 1920s, paint used to inscribe the numbers on watch dials was composed of a luminescent (glow-in-the-dark) mixture. The powdered-paint base was a mixture of radium salts and zinc sulfide. As the paint was mixed, the powdered base became airbo ...
... Setting/Chemistry, and your knowledge of chemistry. In the 1920s, paint used to inscribe the numbers on watch dials was composed of a luminescent (glow-in-the-dark) mixture. The powdered-paint base was a mixture of radium salts and zinc sulfide. As the paint was mixed, the powdered base became airbo ...
New Title
... is it represented in the chemical equation for the reaction? 4. The substances you have at the beginning of a chemical reaction are called the 5. The substances you have when a chemical reaction is complete are called the 6. What do you read the arrow in a chemical equation as meaning? 7. Label each ...
... is it represented in the chemical equation for the reaction? 4. The substances you have at the beginning of a chemical reaction are called the 5. The substances you have when a chemical reaction is complete are called the 6. What do you read the arrow in a chemical equation as meaning? 7. Label each ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.