chemistry paper 1
... Members of higher molecular mass are often used to make soap. The first few members are often used to make polymers. The members can commonly react with hydrogen halides to give halohydrocarbons. A. ...
... Members of higher molecular mass are often used to make soap. The first few members are often used to make polymers. The members can commonly react with hydrogen halides to give halohydrocarbons. A. ...
chem - CBSE Guess
... Rancidity: The oily and fatty food oxidizes and give bad smell and test is called rancidity.Preventatioin:By adding antioxidant which slow down the process of oxidation.2. Vaccum packing,3Flusing N2 gas in chips packets.3.Refrigeration. Q.Explain the various types of reactions with one example of ea ...
... Rancidity: The oily and fatty food oxidizes and give bad smell and test is called rancidity.Preventatioin:By adding antioxidant which slow down the process of oxidation.2. Vaccum packing,3Flusing N2 gas in chips packets.3.Refrigeration. Q.Explain the various types of reactions with one example of ea ...
Gr. 11 Chemistry Student Workbook (Spring 2016)
... One of the most important educational skills you can develop is how to monitor and track your own learning. Either at the end of class or at home, you will complete a daily entry in your learning log. Written Work: Use our marking scheme for daily class work (out of 5) to assess your written work. W ...
... One of the most important educational skills you can develop is how to monitor and track your own learning. Either at the end of class or at home, you will complete a daily entry in your learning log. Written Work: Use our marking scheme for daily class work (out of 5) to assess your written work. W ...
acid
... The forces holding an ionic compound together are the strong electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a par ...
... The forces holding an ionic compound together are the strong electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a par ...
IIT-JEE - Brilliant Public School Sitamarhi
... Point defects: When ions or atoms do not hold the theoretical position, this is called point defect. Point defects are of two types: Stoichiometric defects: Schottky defect: Due to missing of ions from lattice point in pairs. Frenkel defect: It is caused due to the creation of lattice vacancy as a r ...
... Point defects: When ions or atoms do not hold the theoretical position, this is called point defect. Point defects are of two types: Stoichiometric defects: Schottky defect: Due to missing of ions from lattice point in pairs. Frenkel defect: It is caused due to the creation of lattice vacancy as a r ...
Chapter 4 Student Presentation
... Mg(OH)2 (s) + HCl (aq) • Molecular Equation: • Mg(OH)2 (s) + 2 HCl (aq) MgCl2 (aq) + 2 H2O (l) • Complete Ionic Equation: • Mg(OH)2 (s) + 2 H1+(aq) + 2 Cl1-(aq) Mg2+(aq) + 2 Cl1-(aq) + 2 H2O (l) • Net Ionic Equation: • Mg(OH)2 (s) + 2 H1+(aq) Mg2+(aq) + 2 H2O (l) ...
... Mg(OH)2 (s) + HCl (aq) • Molecular Equation: • Mg(OH)2 (s) + 2 HCl (aq) MgCl2 (aq) + 2 H2O (l) • Complete Ionic Equation: • Mg(OH)2 (s) + 2 H1+(aq) + 2 Cl1-(aq) Mg2+(aq) + 2 Cl1-(aq) + 2 H2O (l) • Net Ionic Equation: • Mg(OH)2 (s) + 2 H1+(aq) Mg2+(aq) + 2 H2O (l) ...
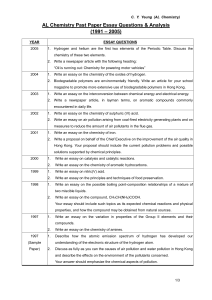
AL Chemistry Past paper essay questions
... Write an essay on amino acids, polypeptides and proteins. Your essay should include the properties of amino acids in aqueous solutions and a method of separation for a mixture of amino acids, as well as the constitution of polypeptides and proteins and their hydrolysis. ...
... Write an essay on amino acids, polypeptides and proteins. Your essay should include the properties of amino acids in aqueous solutions and a method of separation for a mixture of amino acids, as well as the constitution of polypeptides and proteins and their hydrolysis. ...
CHEMISTRY
... 3. An understanding of metric prefixes and their meanings is crucial for conversions between units. I use dimensional analysis to do metric-metric conversions, stressing that many different types of calculations can be done using dimensional analysis. I will also intentionally set up a problem incor ...
... 3. An understanding of metric prefixes and their meanings is crucial for conversions between units. I use dimensional analysis to do metric-metric conversions, stressing that many different types of calculations can be done using dimensional analysis. I will also intentionally set up a problem incor ...
15equil1pp
... An increase in temperature is used to speed up chemical reactions but it can have an undesired effect when the reaction is reversible and exothermic. In this case you get to the equilibrium position quicker but with a reduced yield because the increased temperature moves the equilibrium to the left. ...
... An increase in temperature is used to speed up chemical reactions but it can have an undesired effect when the reaction is reversible and exothermic. In this case you get to the equilibrium position quicker but with a reduced yield because the increased temperature moves the equilibrium to the left. ...
Thermodynamics: Entropy and Free Energy
... Another point about spontaneous processes is that they may require an initial input of energy to begin the process. For example, the reaction of hydrogen and oxygen requires an initial input of energy to overcome the energy of activation (Figure 19.1.8). Once the reaction begins, however, it continu ...
... Another point about spontaneous processes is that they may require an initial input of energy to begin the process. For example, the reaction of hydrogen and oxygen requires an initial input of energy to overcome the energy of activation (Figure 19.1.8). Once the reaction begins, however, it continu ...
(+1) + - Edublogs
... We need an oxidizing agent to convert all the iron from Fe2+ to Fe3+. Primary standard A material that is available in pure form. ...
... We need an oxidizing agent to convert all the iron from Fe2+ to Fe3+. Primary standard A material that is available in pure form. ...
Document
... f) Exothermic reaction and endothermic reaction: On the basis of energy changes during chemical reaction, they can be classified as ...
... f) Exothermic reaction and endothermic reaction: On the basis of energy changes during chemical reaction, they can be classified as ...
Chemistry - Birkenhead School
... The three states of matter can be represented by a simple model. In this model, particles are represented by small solid spheres. Particle theory can help to explain melting, boiling, freezing and condensing. The amount of energy needed to change state from solid to liquid and from liquid to gas dep ...
... The three states of matter can be represented by a simple model. In this model, particles are represented by small solid spheres. Particle theory can help to explain melting, boiling, freezing and condensing. The amount of energy needed to change state from solid to liquid and from liquid to gas dep ...
SUPPORT MATERIAL CLASS – X(science) FIRST TERM
... f) Exothermic reaction and endothermic reaction: On the basis of energy changes during chemical reaction, they can be classified as ...
... f) Exothermic reaction and endothermic reaction: On the basis of energy changes during chemical reaction, they can be classified as ...
Fundamentals Diagnostic Quiz
... d) A compound is a specific combination of atoms of more than one element. e) In a chemical reaction, atoms are neither created nor destroyed; they exchange partners to produce new substances. ...
... d) A compound is a specific combination of atoms of more than one element. e) In a chemical reaction, atoms are neither created nor destroyed; they exchange partners to produce new substances. ...
14.1 Dynamic Equilibrium, Keq , and the Mass Action Expression
... When making assumptions, if a reaction has a relatively small keq and a relatively large initial reactant concentration, then the concentration change (x) can often be neglected without introducing significant error. This does not mean x = 0, because then this would mean there is no reaction. It mea ...
... When making assumptions, if a reaction has a relatively small keq and a relatively large initial reactant concentration, then the concentration change (x) can often be neglected without introducing significant error. This does not mean x = 0, because then this would mean there is no reaction. It mea ...
Gases - chemmybear.com
... It has the largest size or volume. It has the strongest attractive forces (van der Waals forces or dipole-dipole interactions). (c) High temperature result in high kinetic energies. This energy overcomes the attractive forces. Low pressure increases the distance between molecules. (So molecules comp ...
... It has the largest size or volume. It has the strongest attractive forces (van der Waals forces or dipole-dipole interactions). (c) High temperature result in high kinetic energies. This energy overcomes the attractive forces. Low pressure increases the distance between molecules. (So molecules comp ...
Exam - Vcaa
... A. Each solution will have the same electrical conductivity. B. Each solution will react completely with 10.0 mL of 0.10 M NaOH solution. C. Each solution will react at the same rate with 1.00 g of magnesium ribbon. D. The concentration of H3O+ ions will be greater in the CH3COOH solution. SECTI ...
... A. Each solution will have the same electrical conductivity. B. Each solution will react completely with 10.0 mL of 0.10 M NaOH solution. C. Each solution will react at the same rate with 1.00 g of magnesium ribbon. D. The concentration of H3O+ ions will be greater in the CH3COOH solution. SECTI ...
Document
... Thermodynamics is the study of energy and its transformations. All chemical changes involve a transfer of energy, be it into the reaction or out of the reaction. Transformed energy in a chemical reaction comes from or forms chemical bonds and is exchanged with the surroundings as heat and/or work. W ...
... Thermodynamics is the study of energy and its transformations. All chemical changes involve a transfer of energy, be it into the reaction or out of the reaction. Transformed energy in a chemical reaction comes from or forms chemical bonds and is exchanged with the surroundings as heat and/or work. W ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.