Chapter 4 Notes
... Example: The atoms in He and N2, for example, have oxidation numbers of 0. 3. The oxidation number of a monatomic ion equals the charge of the ion. Example: oxidation number of Na+ is +1; the oxidation number of N3- is -3. 4. The oxidation number of oxygen in compounds is usually ...
... Example: The atoms in He and N2, for example, have oxidation numbers of 0. 3. The oxidation number of a monatomic ion equals the charge of the ion. Example: oxidation number of Na+ is +1; the oxidation number of N3- is -3. 4. The oxidation number of oxygen in compounds is usually ...
9/11/01
... amounts of CS2 and O2? - reaction did not go to completion - several competing reactions involving CS2 and/or O2 were going on at the same time. Theoretical yield – the amount of product we should get when a reactant is completely consumed according to our chemical equation Actual yield – the amount ...
... amounts of CS2 and O2? - reaction did not go to completion - several competing reactions involving CS2 and/or O2 were going on at the same time. Theoretical yield – the amount of product we should get when a reactant is completely consumed according to our chemical equation Actual yield – the amount ...
for the exam on 14 feb
... a. AgI in aqueous NaCN to form Ag(CN)216.108 Will a precipitate of BaSO4 form when 100 mL of 4.0 * 10-3 M BaCl2 and 300 mL of 6.0 * 10-4 M Na2SO4 are mixed? Explain. 16.109 Will a precipitate of PbCl2 form on mixing equal volumes of 0.010 M Pb(NO3)2 and 0.010 M HCl? Explain. What minimum Cl- concent ...
... a. AgI in aqueous NaCN to form Ag(CN)216.108 Will a precipitate of BaSO4 form when 100 mL of 4.0 * 10-3 M BaCl2 and 300 mL of 6.0 * 10-4 M Na2SO4 are mixed? Explain. 16.109 Will a precipitate of PbCl2 form on mixing equal volumes of 0.010 M Pb(NO3)2 and 0.010 M HCl? Explain. What minimum Cl- concent ...
Ch. 12 Stoichiometry
... How many molecules of NH3 are needed to produce 2.34 x 1022 molecules of N2F4? How many grams of HF are produced from a reaction of 4.56 x 1023 molecules of F2 with excess NH3? What volume of HF, at STP, can be produced from 345g of NH3? How many molecules of N2F4 can be produce from 45.6L of F2 , a ...
... How many molecules of NH3 are needed to produce 2.34 x 1022 molecules of N2F4? How many grams of HF are produced from a reaction of 4.56 x 1023 molecules of F2 with excess NH3? What volume of HF, at STP, can be produced from 345g of NH3? How many molecules of N2F4 can be produce from 45.6L of F2 , a ...
Learning Outcomes Leaving Certificate Chemistry
... 1.4 Electronic Structure of Atoms (11 class periods) By the end of this section pupils should be able define and explain energy levels in atoms describe the organization of particles in atoms of elements numbers 1-20 classify the first twenty elements in the periodic table on the basis of the number ...
... 1.4 Electronic Structure of Atoms (11 class periods) By the end of this section pupils should be able define and explain energy levels in atoms describe the organization of particles in atoms of elements numbers 1-20 classify the first twenty elements in the periodic table on the basis of the number ...
(MDCAT) 2017 - University Of Health Sciences Lahore
... i) Describe metallic bonding in terms of positive ions surrounded by mobile electrons (sea of electrons). j) Describe, interpret and/or predict the effect of different types of bonding (ionic bonding; covalent bonding; hydrogen bonding; Van der Waal’s forces and metallic bonding) on the physical pro ...
... i) Describe metallic bonding in terms of positive ions surrounded by mobile electrons (sea of electrons). j) Describe, interpret and/or predict the effect of different types of bonding (ionic bonding; covalent bonding; hydrogen bonding; Van der Waal’s forces and metallic bonding) on the physical pro ...
Principles of Chemistry 1 and 2 Notes
... - Valence shell electrons are electrons found in the outermost shell. (# of valence electrons = # of the group in the periodic table) - Valence electrons are the only electrons involved in the chemical reactions. VSEPR: - Valence electron pairs repel each other. - In a polyatomic molecule, the repul ...
... - Valence shell electrons are electrons found in the outermost shell. (# of valence electrons = # of the group in the periodic table) - Valence electrons are the only electrons involved in the chemical reactions. VSEPR: - Valence electron pairs repel each other. - In a polyatomic molecule, the repul ...
Chapter 2: Mass Relations in Formulas, Chemical Reactions, and
... equation is the number of atoms, ions, formula units or molecules associated with each substance. The number in front of each substance is called the stoichiometric coefficients or more simply the coefficient. The bulk of this information is often referred to as the stoichiometry of the chemical rea ...
... equation is the number of atoms, ions, formula units or molecules associated with each substance. The number in front of each substance is called the stoichiometric coefficients or more simply the coefficient. The bulk of this information is often referred to as the stoichiometry of the chemical rea ...
x - SharpSchool
... equilibrium with only partial conversion of reactants to products. Initially, 2.00 mol of each reactant is placed in the vessel. Kc for this reaction is 4.20 at 900C. Calculate the concentration of each substance at equilibrium. ...
... equilibrium with only partial conversion of reactants to products. Initially, 2.00 mol of each reactant is placed in the vessel. Kc for this reaction is 4.20 at 900C. Calculate the concentration of each substance at equilibrium. ...
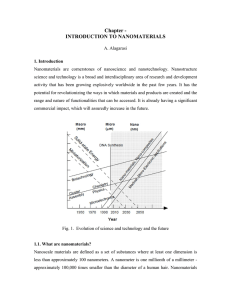
Chapter - INTRODUCTION TO NANOMATERIALS
... 4. Drying of the gel, when water and other volatile liquids are removed from the gel network. This process is complicated due to fundamental changes in the structure of the gel. The drying process has itself been broken into four distinct steps: (i) the constant rate period, (ii) the critical point, ...
... 4. Drying of the gel, when water and other volatile liquids are removed from the gel network. This process is complicated due to fundamental changes in the structure of the gel. The drying process has itself been broken into four distinct steps: (i) the constant rate period, (ii) the critical point, ...
Chemistry booklet
... What about the following PHOSPHO-GLYCERIDE molecule containing both hydrophilic ( water-loving) and hydro-phobic ( water-hating ) regions ( termed an amphi-philic molecule) ? ...
... What about the following PHOSPHO-GLYCERIDE molecule containing both hydrophilic ( water-loving) and hydro-phobic ( water-hating ) regions ( termed an amphi-philic molecule) ? ...
Review on N acylation reaction
... such as Vitamins, agrochemicals, Xanthenes, and in combinatorial peptide synthesis. Literature survey reveals that various drugs such as Penicillin (antibacterial), pyrazineamide (anti tubercular) possess their specific activities due to presence of amide linkage in their structures1. In a typical N ...
... such as Vitamins, agrochemicals, Xanthenes, and in combinatorial peptide synthesis. Literature survey reveals that various drugs such as Penicillin (antibacterial), pyrazineamide (anti tubercular) possess their specific activities due to presence of amide linkage in their structures1. In a typical N ...
Chemistry - Plymouth Public Schools
... MA CHM 4.6 Name and write the chemical formulas for simple ionic and molecular compounds, including those that contain the polyatomic ions: ammonium, carbonate, hydroxide, nitrate, phosphate, and sulfate. Chemical Reactions and Stoichiometry Central Concepts: In a chemical reaction, one or more reac ...
... MA CHM 4.6 Name and write the chemical formulas for simple ionic and molecular compounds, including those that contain the polyatomic ions: ammonium, carbonate, hydroxide, nitrate, phosphate, and sulfate. Chemical Reactions and Stoichiometry Central Concepts: In a chemical reaction, one or more reac ...
Chapter 16: Energy and Chemical Change
... Kinetic energy is energy of motion. You can observe kinetic energy in the motion of people and objects all around you. The potential energy of the dammed water is converted to kinetic energy as the dam gates are opened and the water flows out. Chemical systems contain both kinetic energy and potenti ...
... Kinetic energy is energy of motion. You can observe kinetic energy in the motion of people and objects all around you. The potential energy of the dammed water is converted to kinetic energy as the dam gates are opened and the water flows out. Chemical systems contain both kinetic energy and potenti ...
Notes
... At 25 °C, the reaction I2(g) + Cl2((g) ' 2 ICl(g) has an equilibrium constant KP = 81.9. Initially a reaction mixture at this temperature contains PI2 = 0.100 atm, PCl2 = 0.100 atm and PICl = 0.100 atm. Calculate the equilibrium partial pressures of I2, Cl2, and ICl. ...
... At 25 °C, the reaction I2(g) + Cl2((g) ' 2 ICl(g) has an equilibrium constant KP = 81.9. Initially a reaction mixture at this temperature contains PI2 = 0.100 atm, PCl2 = 0.100 atm and PICl = 0.100 atm. Calculate the equilibrium partial pressures of I2, Cl2, and ICl. ...
Chapter 3
... It is important to know the mass of the atoms especially for the lab work. However; atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it ...
... It is important to know the mass of the atoms especially for the lab work. However; atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it ...
Chapter 13
... shift to reach equilibrium, we compare the values of Q and K. There are three possible cases: 1.Q is equal to K. The system is at equilibrium; no shift will occur. 2.Q is greater than K. The system shifts to the left, consuming products and forming reactants, until equilibrium is achieved. 3.Q is le ...
... shift to reach equilibrium, we compare the values of Q and K. There are three possible cases: 1.Q is equal to K. The system is at equilibrium; no shift will occur. 2.Q is greater than K. The system shifts to the left, consuming products and forming reactants, until equilibrium is achieved. 3.Q is le ...
Practice Qs - Unit 6a
... They all contain metals w/ more than 1 possible charge (oxidation state). + ion: always first (element name or ammonium Charge of metal ion goes as Roman numeral in ( ) - ion: second (name on Table E or root / ide ending) 10. Write IUPAC names the following ionic compounds. Name ...
... They all contain metals w/ more than 1 possible charge (oxidation state). + ion: always first (element name or ammonium Charge of metal ion goes as Roman numeral in ( ) - ion: second (name on Table E or root / ide ending) 10. Write IUPAC names the following ionic compounds. Name ...
The Major Classes of Chemical Reactions
... Chemists use three types of equations to represent aqueous ionic reactions: molecular, total ionic, and net ionic equations. As you’ll see in the two types of ionic equations, by balancing the atoms, we also balance the charges. Let’s examine a reaction to see what each of these equations shows. Whe ...
... Chemists use three types of equations to represent aqueous ionic reactions: molecular, total ionic, and net ionic equations. As you’ll see in the two types of ionic equations, by balancing the atoms, we also balance the charges. Let’s examine a reaction to see what each of these equations shows. Whe ...
day_3_main_lecture - the Essentially Science Wiki!
... – How many moles of H2O are produced from the reaction of 2 moles of H2? – How many moles of O2 are required to produce 4 moles of H2O? ...
... – How many moles of H2O are produced from the reaction of 2 moles of H2? – How many moles of O2 are required to produce 4 moles of H2O? ...
F:\Users\Steven\Documents\Chemistry\CHEM120\Problem Set
... b) Calculate the final concentration of the silver after all the precipitate (solid) has formed. 2) When 75 mL of 0.20M Na3PO4 is added to 125 mL of 0.30 M Zn(NO3)2 a white solid forms. a) Please write the NET ionic reaction that occurred. b) How many grams of solid were made? c) What is the concent ...
... b) Calculate the final concentration of the silver after all the precipitate (solid) has formed. 2) When 75 mL of 0.20M Na3PO4 is added to 125 mL of 0.30 M Zn(NO3)2 a white solid forms. a) Please write the NET ionic reaction that occurred. b) How many grams of solid were made? c) What is the concent ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.