Chemistry - Gildredge House
... represent the structure and formula of organic molecules. Reaction mechanisms are also studied, which enable organic reactions to be explained in a step-by-step process. Content: ...
... represent the structure and formula of organic molecules. Reaction mechanisms are also studied, which enable organic reactions to be explained in a step-by-step process. Content: ...
Introductory Chemistry I
... 3. Which of the following net ionic equations is completely correct? a. K+(aq) + BaCl2(aq) = Ba2+(aq) + KCl(s) b. Ag+(aq) + Cl-(aq) = AgCl(s) c. HCl(aq) + KOH(aq) = H2O(l) + Cl-aq) + K+(aq) d. Cu(s) + SO3-2(aq) + 2 H+(aq) = Cu2+ + SO2(g) + H2O(l) e. Zn + 2 Cl- = ZnCl2 (s) 4. The maximum number of el ...
... 3. Which of the following net ionic equations is completely correct? a. K+(aq) + BaCl2(aq) = Ba2+(aq) + KCl(s) b. Ag+(aq) + Cl-(aq) = AgCl(s) c. HCl(aq) + KOH(aq) = H2O(l) + Cl-aq) + K+(aq) d. Cu(s) + SO3-2(aq) + 2 H+(aq) = Cu2+ + SO2(g) + H2O(l) e. Zn + 2 Cl- = ZnCl2 (s) 4. The maximum number of el ...
File
... elements with respect to their position on the periodic table? a. Increases across a period; increases down a group. b. Increases across a period; decreases down a group. c. Decreases across a period; increases down a group. d. Decreases across a period; decreases down a group. ...
... elements with respect to their position on the periodic table? a. Increases across a period; increases down a group. b. Increases across a period; decreases down a group. c. Decreases across a period; increases down a group. d. Decreases across a period; decreases down a group. ...
S - Valdosta State University
... property of the system. Often at equilibrium. – Here entropy can be obtained at any T by measuring the heat required rise the temperature from 0K, with the slow addition of heat in very small amounts. – DS = qrev/T ...
... property of the system. Often at equilibrium. – Here entropy can be obtained at any T by measuring the heat required rise the temperature from 0K, with the slow addition of heat in very small amounts. – DS = qrev/T ...
1 - Study Hungary
... C: the atomic number increases by 1 and the mass number doesn’t change. D: the loss of a neutron decreases the mass number by 1 and the charge by 1. E: the loss of a proton decreases the mass number by 1 and increases the ...
... C: the atomic number increases by 1 and the mass number doesn’t change. D: the loss of a neutron decreases the mass number by 1 and the charge by 1. E: the loss of a proton decreases the mass number by 1 and increases the ...
H3AsO4 + 3 I- + 2 H3O+ H3AsO3 + I3- + H2O
... the result of electrostatic forces between cations and anions; covalent bonds form when electrons are shared between non-metal atoms; and metallic bonds, which bind metal cations with mutually shared valence electrons. Bonds involve the interaction of valence electrons, which are represented by elec ...
... the result of electrostatic forces between cations and anions; covalent bonds form when electrons are shared between non-metal atoms; and metallic bonds, which bind metal cations with mutually shared valence electrons. Bonds involve the interaction of valence electrons, which are represented by elec ...
JF Physical Chemistry 2010-2011. JF CH 1101: Introduction to
... 3. Calculate the work done by a system in which a reaction results in the formation of 1 mol CO2 gas at 298 K and 100 kPa. Assume that CO2 behaves as an ideal gas. 4. Calculate the work done when 1 mol Ar gas confined in a cylinder of volume 1 dm3 at 298 K expands isothermally and reversibly to 2 ...
... 3. Calculate the work done by a system in which a reaction results in the formation of 1 mol CO2 gas at 298 K and 100 kPa. Assume that CO2 behaves as an ideal gas. 4. Calculate the work done when 1 mol Ar gas confined in a cylinder of volume 1 dm3 at 298 K expands isothermally and reversibly to 2 ...
chemistry 11 exam review
... 5. What is the new volume of 750.0 mL of gas at 700.0 torr when the pressure is changed to 800.0 torr? (656.3 torr) 6. 45 mL of gas at 15C and 790 torr is changed to 23C and 810 torr. What is the new volume? (45 mL) 7. 175 mL of gas at –30.0C and 2.57 atm is changed to standard conditions. What i ...
... 5. What is the new volume of 750.0 mL of gas at 700.0 torr when the pressure is changed to 800.0 torr? (656.3 torr) 6. 45 mL of gas at 15C and 790 torr is changed to 23C and 810 torr. What is the new volume? (45 mL) 7. 175 mL of gas at –30.0C and 2.57 atm is changed to standard conditions. What i ...
File
... 3.C.2 Net changes in energy for a chemical reaction can be endothermic or exothermic. 5.A.1 Temperature is a measure of the average kinetic energy of atoms and molecules. 5.A.2 The process of kinetic energy transfer at the particulate scale is referred to in this course as heat transfer, and t ...
... 3.C.2 Net changes in energy for a chemical reaction can be endothermic or exothermic. 5.A.1 Temperature is a measure of the average kinetic energy of atoms and molecules. 5.A.2 The process of kinetic energy transfer at the particulate scale is referred to in this course as heat transfer, and t ...
BIOL 157 * BIOLOGICAL CHEMISTRY Lecture 6
... • ii. What is the order of reaction with respect to A and B? • iii. What is the overall order of reaction? • iv. Calculate the rate constant for the reaction ...
... • ii. What is the order of reaction with respect to A and B? • iii. What is the overall order of reaction? • iv. Calculate the rate constant for the reaction ...
2013 us national chemistry olympiad
... c. Recent calculations predict that the two structures that are lowest in energy differ by about 0.2 kJ/mol. Identify the second lowest energy structure and justify your answer. d. Nuclear magnetic resonance (NMR) spectroscopy can be used to distinguish between atoms in different environments in a m ...
... c. Recent calculations predict that the two structures that are lowest in energy differ by about 0.2 kJ/mol. Identify the second lowest energy structure and justify your answer. d. Nuclear magnetic resonance (NMR) spectroscopy can be used to distinguish between atoms in different environments in a m ...
Word - chemmybear.com
... Note: If you were asked to write the overall equation, you would flip the Tl+ + e Tl equation around and multiply it by 3. However, you do NOT change the Ereduction because these have been determined by comparing every half cell to the same standard half cell. The e’s have already been accounte ...
... Note: If you were asked to write the overall equation, you would flip the Tl+ + e Tl equation around and multiply it by 3. However, you do NOT change the Ereduction because these have been determined by comparing every half cell to the same standard half cell. The e’s have already been accounte ...
Production of Materials by Jimmy Huang
... Ethanol is a good solvent for both polar and non-polar substances due to its structure. The nonpolar CH3 end can dissolve non-polar substances through dispersion forces, while the OH end can dissolve polar substances due to the powerful hydrogen bonding. For these properties, ethanol is widely used ...
... Ethanol is a good solvent for both polar and non-polar substances due to its structure. The nonpolar CH3 end can dissolve non-polar substances through dispersion forces, while the OH end can dissolve polar substances due to the powerful hydrogen bonding. For these properties, ethanol is widely used ...
Organic Chemistry and Medicine
... Organic chemistry is the one course EVERYONE has heard of and many DREAD taking Organic chemistry is usually a “make it or break it” class for pre-med students ...
... Organic chemistry is the one course EVERYONE has heard of and many DREAD taking Organic chemistry is usually a “make it or break it” class for pre-med students ...
unit (4) calculations and chemical reactions
... Some examples are shown below: 2Mg(s) + O2(g) → 2MgO(s) 2Na(s) + Cl2(g) → 2NaCl(s) SO3(g) + H2O(l) → H2SO4(aq) II) Decomposition Reactions In a decomposition reaction, a reactant splits into two or more simpler products. The general form of the reaction is (AB → A + B). Some examples are shown below ...
... Some examples are shown below: 2Mg(s) + O2(g) → 2MgO(s) 2Na(s) + Cl2(g) → 2NaCl(s) SO3(g) + H2O(l) → H2SO4(aq) II) Decomposition Reactions In a decomposition reaction, a reactant splits into two or more simpler products. The general form of the reaction is (AB → A + B). Some examples are shown below ...
Balancing ANY chemical Equation
... - Involve the exchange or “changing out” of certain ions for others. - Positive ions switch places and get new “partners” or negative ions. - The new products are formed based on the charges of the ions being put together . Ex: BaCl2 + Na2SO4 2NaCl + BaSO4 ...
... - Involve the exchange or “changing out” of certain ions for others. - Positive ions switch places and get new “partners” or negative ions. - The new products are formed based on the charges of the ions being put together . Ex: BaCl2 + Na2SO4 2NaCl + BaSO4 ...
Bk2P06EE
... reaction that gives out heat. Since the crystallization of potassium nitrate is an exothermic process, it is favoured and crystals formed. Ca2+(aq) + SO42(aq) CaSO4(s) When concentrated sodium sulphate solution is added to a saturated solution of calcium sulphate, the concentration of sulphate ions ...
... reaction that gives out heat. Since the crystallization of potassium nitrate is an exothermic process, it is favoured and crystals formed. Ca2+(aq) + SO42(aq) CaSO4(s) When concentrated sodium sulphate solution is added to a saturated solution of calcium sulphate, the concentration of sulphate ions ...
Lecture 1: RDCH 710 Introduction
... Can also use Cs2NpO2Cl4 and Cs3NpO2Cl4 LiCl/KCl as electrolyte at 723 K NpC reduction with Ta followed by volatilization of Np Electrodepostion from aqueous solution Amalgamation with Hg from 1 M CH3COOH and 0.3 M CH3COONa at pH 3.5 Distillation to remove Hg ...
... Can also use Cs2NpO2Cl4 and Cs3NpO2Cl4 LiCl/KCl as electrolyte at 723 K NpC reduction with Ta followed by volatilization of Np Electrodepostion from aqueous solution Amalgamation with Hg from 1 M CH3COOH and 0.3 M CH3COONa at pH 3.5 Distillation to remove Hg ...
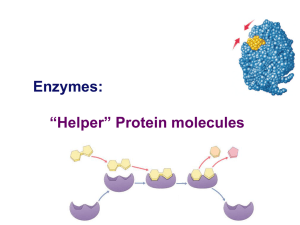
Enzymes: “Helper” Protein molecules
... Enzymes are not changed by the reaction used only temporarily – like a taxi re-used again for the same reaction with other molecules very little enzyme needed to help in many reactions ...
... Enzymes are not changed by the reaction used only temporarily – like a taxi re-used again for the same reaction with other molecules very little enzyme needed to help in many reactions ...
Atomic Theory
... When energy is applied to specific (individual) elements they emit a spectrum which only contains emissions of particular s. A line spectrum is not continuous. Each element has its own characteristic line spectrum. Hydrogen spectrum- it consists of discrete lines that converge towards the high ene ...
... When energy is applied to specific (individual) elements they emit a spectrum which only contains emissions of particular s. A line spectrum is not continuous. Each element has its own characteristic line spectrum. Hydrogen spectrum- it consists of discrete lines that converge towards the high ene ...
Prescribed Practicals
... yellow. This means that a solution with pH ≤ 3.1 will be red and pH ≥ 4.4 will be yellow. However, if the pH of the solution is between 3.1 and 4.4, the solution will appear orange. This is known as its transition colour. ...
... yellow. This means that a solution with pH ≤ 3.1 will be red and pH ≥ 4.4 will be yellow. However, if the pH of the solution is between 3.1 and 4.4, the solution will appear orange. This is known as its transition colour. ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.