AP Chemistry
... When you measure something, however, you obtain a number that is not exact. For example, you can determine that a beaker has a mass of 250 g by weighing it on a scale. Using a different scale might give you a mass of 249.9 g for the same beaker. Yet another scale might report the mass as 249.89 g. W ...
... When you measure something, however, you obtain a number that is not exact. For example, you can determine that a beaker has a mass of 250 g by weighing it on a scale. Using a different scale might give you a mass of 249.9 g for the same beaker. Yet another scale might report the mass as 249.89 g. W ...
1 Lecture 11. Redox Chemistry Many elements in the periodic table
... There is an “ideal” sequence of redox reactions based on the energy available. In this sequence, organic matter is combusted in order by O2, NO3, MnO2, Fe2O3 then SO42- to provide this energy. Most of these reactions have slow kinetics if left to occur on their own. Bacteria mediate most of these re ...
... There is an “ideal” sequence of redox reactions based on the energy available. In this sequence, organic matter is combusted in order by O2, NO3, MnO2, Fe2O3 then SO42- to provide this energy. Most of these reactions have slow kinetics if left to occur on their own. Bacteria mediate most of these re ...
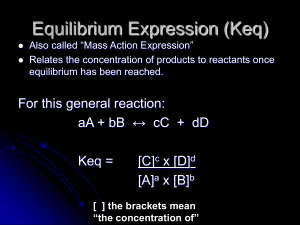
Equilibrium Expression (Keq)
... (The is a unitless number and is unique to that reaction.) The only thing that can change the value of Keq is a change in temperature. ...
... (The is a unitless number and is unique to that reaction.) The only thing that can change the value of Keq is a change in temperature. ...
Chapter 6 - Sites @ Suffolk University
... This simple and useful concept of gram molecular mass is often abbreviated to the term "mole," which conjures up images of furry animals in the minds of the uninitiated. Most often, for instance, chemists will say that water has 18 grams per mole. There is a technical problem with the use of this te ...
... This simple and useful concept of gram molecular mass is often abbreviated to the term "mole," which conjures up images of furry animals in the minds of the uninitiated. Most often, for instance, chemists will say that water has 18 grams per mole. There is a technical problem with the use of this te ...
Experimental Chemistry I
... that required to neutralize the analyte (based on the stoichiometric relation between titrant and analyte). Procedure: Clean 50mL burette with deionized water and 1. rinse burette with calibration solution (1.000M) NaOH and fill it up to zero-mark; close stopcock before filling with titrant and clam ...
... that required to neutralize the analyte (based on the stoichiometric relation between titrant and analyte). Procedure: Clean 50mL burette with deionized water and 1. rinse burette with calibration solution (1.000M) NaOH and fill it up to zero-mark; close stopcock before filling with titrant and clam ...
2009 - NESACS
... 51. Carbon in the Universe comes from 3 He-4 nuclei fusing to create a C-12 atom—A very slow process requiring 100 million K deep inside giant red star core where H is all consumed and He is in abundance. Unstable Be-8 is crucial in creating C-12 but for a split second, 2 He−4 particles fuse to make ...
... 51. Carbon in the Universe comes from 3 He-4 nuclei fusing to create a C-12 atom—A very slow process requiring 100 million K deep inside giant red star core where H is all consumed and He is in abundance. Unstable Be-8 is crucial in creating C-12 but for a split second, 2 He−4 particles fuse to make ...
Equilibrium Review True/False Indicate whether the statement is
... NAT: UCP.4 | B.3 | B.6 2. ANS: T PTS: 1 DIF: Bloom's Level 2|DOK 1 NAT: UCP.2 | UCP.4 | B.3 3. ANS: F Heat is given out in the forward reaction of an exothermic reversible reaction. Therefore, exothermic reactions are not favored at increased (high) temperature. PTS: 1 DIF: Bloom's Level 2|DOK 1 NAT ...
... NAT: UCP.4 | B.3 | B.6 2. ANS: T PTS: 1 DIF: Bloom's Level 2|DOK 1 NAT: UCP.2 | UCP.4 | B.3 3. ANS: F Heat is given out in the forward reaction of an exothermic reversible reaction. Therefore, exothermic reactions are not favored at increased (high) temperature. PTS: 1 DIF: Bloom's Level 2|DOK 1 NAT ...
CHEMISTRY
... using structural formulas, write the overall equation for the reaction between A and B to form Captopril®. ...
... using structural formulas, write the overall equation for the reaction between A and B to form Captopril®. ...
A Semianalytical Method for Predicting Primary and Secondary
... condition (Eq. 4) at y 5 1 (i.e., f1 5 f2 5 fb) T ...
... condition (Eq. 4) at y 5 1 (i.e., f1 5 f2 5 fb) T ...
AP Chemistry - West Bloomfield School District
... In a certain experiment, 6.00 g of aluminum is burned in 24.0 g of bromine. What is the maximum amount of aluminum bromide that can be produced? 67. Acid-base neutralization reactions are very common in industrial processes. This is the reaction of sulfuric acid with sodium hydroxide: H2SO4 (aq) + 2 ...
... In a certain experiment, 6.00 g of aluminum is burned in 24.0 g of bromine. What is the maximum amount of aluminum bromide that can be produced? 67. Acid-base neutralization reactions are very common in industrial processes. This is the reaction of sulfuric acid with sodium hydroxide: H2SO4 (aq) + 2 ...
9701/04 - StudyGuide.PK
... (b) AgBr is only sparingly soluble in water. The [Ag+] in a saturated solution of AgBr can be estimated by measuring the Ecell of the following cell. C ...
... (b) AgBr is only sparingly soluble in water. The [Ag+] in a saturated solution of AgBr can be estimated by measuring the Ecell of the following cell. C ...
Theoretical problems
... Preparatory Problems, Theoretical contaminations. The reaction of sulfuric acid with colemanite takes place in two steps: In the first step colemanite is dissolved in sulfuric acid forming the calcium(II) ion and boric acid. In the second step, calcium sulfate, formed from Ca 2+ and SO4 ions, pre ...
... Preparatory Problems, Theoretical contaminations. The reaction of sulfuric acid with colemanite takes place in two steps: In the first step colemanite is dissolved in sulfuric acid forming the calcium(II) ion and boric acid. In the second step, calcium sulfate, formed from Ca 2+ and SO4 ions, pre ...
Fundamentals Diagnostic Quiz
... 19. All of the following statements are true regarding the nuclear model of the atom, except: a) The positive charge is densely found in the center of the atom, while the negatively charged electrons exist in a diffuse cloud outside the nucleus. b) Most of the space of an atom is empty space. *c) Th ...
... 19. All of the following statements are true regarding the nuclear model of the atom, except: a) The positive charge is densely found in the center of the atom, while the negatively charged electrons exist in a diffuse cloud outside the nucleus. b) Most of the space of an atom is empty space. *c) Th ...
Exemplar Paper
... Ca(OCl)2(s) + 2HCl(aq) → Ca(OH)2(aq) + 2Cl2(g) alculate the mass of calcium hypochlorite that would be needed to C produce 0·096 litres of chlorine gas. (Take the molar volume of chlorine gas to be 24 litres mol−1.) ...
... Ca(OCl)2(s) + 2HCl(aq) → Ca(OH)2(aq) + 2Cl2(g) alculate the mass of calcium hypochlorite that would be needed to C produce 0·096 litres of chlorine gas. (Take the molar volume of chlorine gas to be 24 litres mol−1.) ...
to view
... How is the vapour pressure of a solvent affected when a non volatile solute is dissolved in it? A1 When a non volatile solute is added to a solvent, its vapour pressure decreases because some of the surface sites are occupied by solute molecules. Thus less space is available for the solvent molecule ...
... How is the vapour pressure of a solvent affected when a non volatile solute is dissolved in it? A1 When a non volatile solute is added to a solvent, its vapour pressure decreases because some of the surface sites are occupied by solute molecules. Thus less space is available for the solvent molecule ...
Line 4: Equation
... These are a little tricky to balance, you just have to have patience. Start with the carbons first. Notice, some of the numbers on line 3 are crossed out. I had to try this equation a couple of different ways. Line 1: organic + oxygen carbon dioxide + water Line 2: C2H6 + oxygen carbon dioxide + ...
... These are a little tricky to balance, you just have to have patience. Start with the carbons first. Notice, some of the numbers on line 3 are crossed out. I had to try this equation a couple of different ways. Line 1: organic + oxygen carbon dioxide + water Line 2: C2H6 + oxygen carbon dioxide + ...
Chemistry (306) - National Evaluation Series
... Which of the following is best accomplished using a mass spectrometer? A. determining the percent abundance of an element's natural isotopes B. determining the triple point of an unknown substance C. determining the reaction rate for a chemical reaction involving a gas D. determining the electronega ...
... Which of the following is best accomplished using a mass spectrometer? A. determining the percent abundance of an element's natural isotopes B. determining the triple point of an unknown substance C. determining the reaction rate for a chemical reaction involving a gas D. determining the electronega ...
Macroscopic electric field and osmotic pressure in ultracentrifugal
... Salt bridges are necessary for the electrochemical measurement of Donnan potentials [14-15]. An incorrect approach would be to measure the voltage between two reversible electrodes without salt bridges, for instance two platinum electrodes, because the potential difference between two reversible ele ...
... Salt bridges are necessary for the electrochemical measurement of Donnan potentials [14-15]. An incorrect approach would be to measure the voltage between two reversible electrodes without salt bridges, for instance two platinum electrodes, because the potential difference between two reversible ele ...
REDOX EQUILIBRIA SL - chemistryatdulwich
... 9.4.1 Explain how a redox reaction is used to produce electricity in a voltaic cell. 9.4.2 State that oxidation occurs at the negative electrode (anode) and reduction occurs at the positive electrode (cathode). ...
... 9.4.1 Explain how a redox reaction is used to produce electricity in a voltaic cell. 9.4.2 State that oxidation occurs at the negative electrode (anode) and reduction occurs at the positive electrode (cathode). ...
Mnemonic Devices - Free WonderKids-e
... - is another weak oxidizing agent. Its mode of action is to decompose into water and atomic oxygen (O). Atomic oxygen normally combines to form molecular . However, in the brief time that is available, atomic oxygen acts as a very good oxidizing agent if it encounters a suitable reactant. Hydrogen p ...
... - is another weak oxidizing agent. Its mode of action is to decompose into water and atomic oxygen (O). Atomic oxygen normally combines to form molecular . However, in the brief time that is available, atomic oxygen acts as a very good oxidizing agent if it encounters a suitable reactant. Hydrogen p ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.