Studies on Transport of Carbonate Ions through a Supported Liquid
... are advantageous because their transports are controlled by their solubility, reactivity and diffusion through a solvent medium. These studies were undertaken in order to investigate the enhanced transport of anions through a liquid membrane with the help of carriers under dynamic and steady-state c ...
... are advantageous because their transports are controlled by their solubility, reactivity and diffusion through a solvent medium. These studies were undertaken in order to investigate the enhanced transport of anions through a liquid membrane with the help of carriers under dynamic and steady-state c ...
Preparation of spherical DDNP study Liu off on a journey
... Influence of ammonia salt crystals diazotization due in ammonia salt filtration process Amount drained liquor, ammonium salt crystals when stout, filtration fast, do not And then washed with water. Because the sodium picramate reduction is an exothermic reaction, the addition point may be scattered ...
... Influence of ammonia salt crystals diazotization due in ammonia salt filtration process Amount drained liquor, ammonium salt crystals when stout, filtration fast, do not And then washed with water. Because the sodium picramate reduction is an exothermic reaction, the addition point may be scattered ...
12 U Chem Review
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
sch4ureview
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
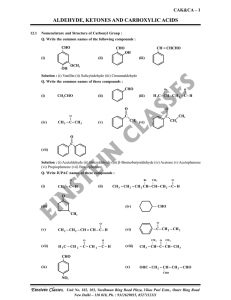
aldehyde, ketones and carboxylic acids
... intermediate captures a proton from the reaction medium to give the electrically neutral product. The net result is addition of Nu– and H+ across the carbon oxygen double bond. Q. Why Aldehydes are more reactive than Ketones ? Solution : There are two reasons for this, they are as follows : 1. Steri ...
... intermediate captures a proton from the reaction medium to give the electrically neutral product. The net result is addition of Nu– and H+ across the carbon oxygen double bond. Q. Why Aldehydes are more reactive than Ketones ? Solution : There are two reasons for this, they are as follows : 1. Steri ...
HEAd START TO A LEVEL CHEMISTRY WORKbOOK
... eg CO is Carbon Monoxide where mon- means one CO 2 is Carbon Dioxide where di- means two SO 2 is Sulphur Dioxide. This could be called Sulphur(IV) Oxide SO 3 is Sulphur Trioxide. This could be called Sulphur (VI) Oxide PCl 3 is Phosphorus Trichloride. This could be called Phosphorus (III) Chloride P ...
... eg CO is Carbon Monoxide where mon- means one CO 2 is Carbon Dioxide where di- means two SO 2 is Sulphur Dioxide. This could be called Sulphur(IV) Oxide SO 3 is Sulphur Trioxide. This could be called Sulphur (VI) Oxide PCl 3 is Phosphorus Trichloride. This could be called Phosphorus (III) Chloride P ...
Lecture 7
... Anomalous or unusual properties of the first member of the group, beryllium 1. Beryllium oxide is amphoteric as base: BeO(s) + 2H3O+(aq) → Be2+(aq) + 3H2O(l) as acid: BeO(s) + 2OH-(aq) + H2O(l) → Be(OH)4-(aq) 2. Beryllium chloride forms a layer lattice rather than an ionic one. In this way it is lik ...
... Anomalous or unusual properties of the first member of the group, beryllium 1. Beryllium oxide is amphoteric as base: BeO(s) + 2H3O+(aq) → Be2+(aq) + 3H2O(l) as acid: BeO(s) + 2OH-(aq) + H2O(l) → Be(OH)4-(aq) 2. Beryllium chloride forms a layer lattice rather than an ionic one. In this way it is lik ...
File - Junior College Chemistry tuition
... Gaseous particle U has an atomic number n and a charge of +1. Gaseous particle V has an atomic number of (n + 1) and is isoelectronic with U. Which of the following statements is always true? ...
... Gaseous particle U has an atomic number n and a charge of +1. Gaseous particle V has an atomic number of (n + 1) and is isoelectronic with U. Which of the following statements is always true? ...
percent composition and formulas
... heating Si in chlorine gas: Si(s) + Cl2(g) SiCl4(l) In one reaction 0.507 mole of SiCl4 is produced. How many moles of molecular chlorine were used in the reaction. ...
... heating Si in chlorine gas: Si(s) + Cl2(g) SiCl4(l) In one reaction 0.507 mole of SiCl4 is produced. How many moles of molecular chlorine were used in the reaction. ...
physical setting chemistry
... This is a test of your knowledge of chemistry. Use that knowledge to answer all questions in this examination. Some questions may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry. You are to answer all questions in all parts of this examination according to the dir ...
... This is a test of your knowledge of chemistry. Use that knowledge to answer all questions in this examination. Some questions may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry. You are to answer all questions in all parts of this examination according to the dir ...
PLACE LABEL HERE Tasmanian Certificate of Education
... All questions should be answered. Answers must be written in the spaces provided on the examination paper. Each question is of equal value and should take about 22 minutes. You should make sure you answer all parts from each question so that all criteria can be assessed. The quality and appropriaten ...
... All questions should be answered. Answers must be written in the spaces provided on the examination paper. Each question is of equal value and should take about 22 minutes. You should make sure you answer all parts from each question so that all criteria can be assessed. The quality and appropriaten ...
Chemical Formulas and their arithmetic
... Several related terms are used to express the mass of one mole of a substance. Molecular weight This is analogous to atomic weight: it is the relative weight of one formula unit of the compound, based on the carbon12 scale. The molecular weight is found by adding atomic weights of all the atoms pre ...
... Several related terms are used to express the mass of one mole of a substance. Molecular weight This is analogous to atomic weight: it is the relative weight of one formula unit of the compound, based on the carbon12 scale. The molecular weight is found by adding atomic weights of all the atoms pre ...
Lectures on Chapter 4, Part 2 Powerpoint 97 Document
... SO32-(aq) SO42-(aq) + 2 e Add water to the reactant side to supply an oxygen and add two protons to the product side that will remain plus the two electrons. SO32-(aq) + H2O(l) SO42-(aq) + 2 H+(aq) + 2 e Reduction: MnO4-(aq) + 3 eMnO2 (s) Add water to the product side to take up the extra oxygen fro ...
... SO32-(aq) SO42-(aq) + 2 e Add water to the reactant side to supply an oxygen and add two protons to the product side that will remain plus the two electrons. SO32-(aq) + H2O(l) SO42-(aq) + 2 H+(aq) + 2 e Reduction: MnO4-(aq) + 3 eMnO2 (s) Add water to the product side to take up the extra oxygen fro ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.