physical setting chemistry
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
Solid-State 23Na Nuclear Magnetic Resonance of Sodium
... ion-ion interaction between Na+ and Br- (2.82 Å), the NaB ion is out of the 18-crown-6 plane by 0.27 Å toward the bromide ion. In contrast, NaA is only 0.07 Å out of the 18-crown-6 plane. Another piece of evidence supporting our assignment is that, although the sodium-oxygen distances around NaB var ...
... ion-ion interaction between Na+ and Br- (2.82 Å), the NaB ion is out of the 18-crown-6 plane by 0.27 Å toward the bromide ion. In contrast, NaA is only 0.07 Å out of the 18-crown-6 plane. Another piece of evidence supporting our assignment is that, although the sodium-oxygen distances around NaB var ...
Stoichiometric Calculations
... Example: When 10 grams of hydrogen react with 3.4 moles of nitrogen gas to make ammonia, which substance would be the limiting reactant? N2(g) + 3H2(g) --> 2NH3(g) Step 1: Convert all values to moles. 10 g H2 x 1 mol H2 = 5 mol H2 2 g H2 Initial Amounts = 5 mol H2, 3.4 mol N2 ...
... Example: When 10 grams of hydrogen react with 3.4 moles of nitrogen gas to make ammonia, which substance would be the limiting reactant? N2(g) + 3H2(g) --> 2NH3(g) Step 1: Convert all values to moles. 10 g H2 x 1 mol H2 = 5 mol H2 2 g H2 Initial Amounts = 5 mol H2, 3.4 mol N2 ...
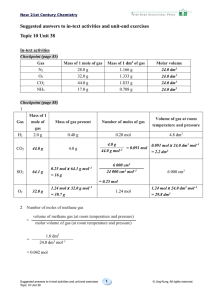
2. Solution Guide to Supplementary Exercises
... Option B — A fuse is a safety device that protects electric circuits from the effects of excessive electric currents. It commonly consists of a current-conducting wire of low melting point. If we try to pass a current higher than the rated value through the wire, it will heat up so much that it melt ...
... Option B — A fuse is a safety device that protects electric circuits from the effects of excessive electric currents. It commonly consists of a current-conducting wire of low melting point. If we try to pass a current higher than the rated value through the wire, it will heat up so much that it melt ...
IPPC S4.03 Inorganic Chemicals Consultation Draft v1.1
... • Internal business or personal use. (This document may be used without restriction for private use or for use within businesses.) • Giving copies to others. (Copies may be made without restriction, providing no charge is made.) If anyone wishes to use this document in any way other than as set out ...
... • Internal business or personal use. (This document may be used without restriction for private use or for use within businesses.) • Giving copies to others. (Copies may be made without restriction, providing no charge is made.) If anyone wishes to use this document in any way other than as set out ...
Geochemistry of Boom Clay pore water at the Mol site
... high-level radioactive waste and spent fuel. The Boom Clay is studied as the reference host rock for methodological studies on the geological disposal of radioactive waste. In many of these studies, an in-depth understanding of the Boom Clay pore water geochemistry is essential. The objective of thi ...
... high-level radioactive waste and spent fuel. The Boom Clay is studied as the reference host rock for methodological studies on the geological disposal of radioactive waste. In many of these studies, an in-depth understanding of the Boom Clay pore water geochemistry is essential. The objective of thi ...
File
... Oxygen and silicon are the two most abundant elements in the earth’s crust, oceans, and atmosphere. Oxygen is found in the atmosphere as O2, in the oceans in H2O, and in the earth’s crust primarily in silicate and carbonate minerals. Because oxygen is everywhere, it is not too surprising that it is ...
... Oxygen and silicon are the two most abundant elements in the earth’s crust, oceans, and atmosphere. Oxygen is found in the atmosphere as O2, in the oceans in H2O, and in the earth’s crust primarily in silicate and carbonate minerals. Because oxygen is everywhere, it is not too surprising that it is ...
Stoichiometry - Social Circle City Schools
... As you learned in Unit 1, atoms are so small and have such small masses that any amount of atoms we would work with would be very hard to count. For example, a piece of aluminum about the size of a pencil eraser contains approximately 2 × 1022 aluminum atoms! The mole (abbreviated mol) is the unit c ...
... As you learned in Unit 1, atoms are so small and have such small masses that any amount of atoms we would work with would be very hard to count. For example, a piece of aluminum about the size of a pencil eraser contains approximately 2 × 1022 aluminum atoms! The mole (abbreviated mol) is the unit c ...
Fundamentals of Combustion
... 1.2.1 Thermal explosion theory . . . . . . . . . . . . . . . . . . 1.2.1.1 One-step reversible kinetics . . . . . . . . . . . . 1.2.1.2 First law of thermodynamics . . . . . . . . . . . 1.2.1.3 Dimensionless form . . . . . . . . . . . . . . . . . 1.2.1.4 Example calculation . . . . . . . . . . . . . ...
... 1.2.1 Thermal explosion theory . . . . . . . . . . . . . . . . . . 1.2.1.1 One-step reversible kinetics . . . . . . . . . . . . 1.2.1.2 First law of thermodynamics . . . . . . . . . . . 1.2.1.3 Dimensionless form . . . . . . . . . . . . . . . . . 1.2.1.4 Example calculation . . . . . . . . . . . . . ...
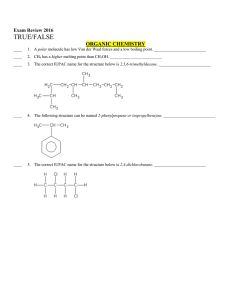
Multiple Choice Exam Review June 2016
... ____ 50. A system at equilibrium means the concentration of reactants is equal to the concentration of products. _______________________________________________________ ____ 51. In a saturated solution of aluminum hydroxide the [Al3+] is always 1/3 the [OH1–]. _________________________ ...
... ____ 50. A system at equilibrium means the concentration of reactants is equal to the concentration of products. _______________________________________________________ ____ 51. In a saturated solution of aluminum hydroxide the [Al3+] is always 1/3 the [OH1–]. _________________________ ...
Atomic and electronic structure of MoS2 nanoparticles
... generally believed that the active sites are located at the edges of the catalyst particles and are associated with the presence of coordinatively unsaturated Mo atoms. 共iii兲 MoS2 is an effective solid lubricant. MoS2 fullerenelike particles have been reported to have very low friction and wear prop ...
... generally believed that the active sites are located at the edges of the catalyst particles and are associated with the presence of coordinatively unsaturated Mo atoms. 共iii兲 MoS2 is an effective solid lubricant. MoS2 fullerenelike particles have been reported to have very low friction and wear prop ...
CHAPTER 12 | The Chemistry of Solids
... Band theory is a model of bonding in which orbitals on many atoms are combined, as in molecular orbital theory, to form a fully or partially filled valence band and an empty conduction band. Solve Yes, by combining the 1s orbitals on many hydrogen atoms at very low temperatures and at high pressures ...
... Band theory is a model of bonding in which orbitals on many atoms are combined, as in molecular orbital theory, to form a fully or partially filled valence band and an empty conduction band. Solve Yes, by combining the 1s orbitals on many hydrogen atoms at very low temperatures and at high pressures ...
Modification of Polycaprolactone by Titania Through a Sol
... As for thermal stability, 10 wt.% TiO2 provided the most favorable outcome in both hybrids for tensile strength, with maximum values at this titania content (Fig. 5). For PCL/TiO2 hybrids, the effect of titania content on the tensile strength is somewhat insignificant because the interfacial force b ...
... As for thermal stability, 10 wt.% TiO2 provided the most favorable outcome in both hybrids for tensile strength, with maximum values at this titania content (Fig. 5). For PCL/TiO2 hybrids, the effect of titania content on the tensile strength is somewhat insignificant because the interfacial force b ...
006 Thermochemistry
... 23. Glycine, C2H5O2N, is important for biological energy. The combustion reaction of glycine is given by the equation: 4C2H5O2N(s) + 9O2(g) 8CO2(g) + 10H2O(l) + 2N2(g) Given that [CO2(g)] = -393.5 kJ/mol and formation of glycine. A. B. C. D. E. ...
... 23. Glycine, C2H5O2N, is important for biological energy. The combustion reaction of glycine is given by the equation: 4C2H5O2N(s) + 9O2(g) 8CO2(g) + 10H2O(l) + 2N2(g) Given that [CO2(g)] = -393.5 kJ/mol and formation of glycine. A. B. C. D. E. ...
edexcel_u4_2010_2013..
... Answer ALL the questions in this section. You should aim to spend no more than 20 minutes on this section. For each question select one answer from A to D and put a cross in the box . and then mark your new answer with If you change your mind, put a line through the box ...
... Answer ALL the questions in this section. You should aim to spend no more than 20 minutes on this section. For each question select one answer from A to D and put a cross in the box . and then mark your new answer with If you change your mind, put a line through the box ...
Nanofibers and Nanoporous Metal Oxides for Gas Sensing
... the most widely agreed and up-to-date assessments of air pollutants on human health, recommending stringent thresholds for air quality. This consequently signifies the need to develop gas sensors with the specifications that meet WHO targets. Additionally, gas sensors are also used in variety of oth ...
... the most widely agreed and up-to-date assessments of air pollutants on human health, recommending stringent thresholds for air quality. This consequently signifies the need to develop gas sensors with the specifications that meet WHO targets. Additionally, gas sensors are also used in variety of oth ...
Moles Workbook
... Atoms are the particles whose symbols are found in the periodic table given in all your examination papers and also on page 113 of this book. You can see there are only about 100 of them. The middle part of the atom, the nucleus, contains one or more protons. It is the number of protons that make th ...
... Atoms are the particles whose symbols are found in the periodic table given in all your examination papers and also on page 113 of this book. You can see there are only about 100 of them. The middle part of the atom, the nucleus, contains one or more protons. It is the number of protons that make th ...
Dynamic Equilibria in Solvent-Mediated Anion, Cation and Ligand
... evidence is given for such a mechanism or assignment. Compared to typical molecular complexes of low to medium molecular weights, many coordination polymers have a perceived low solubility in most conventional solvents and so are often described as “insoluble”.[17, 18, 29] In addition, it has been ...
... evidence is given for such a mechanism or assignment. Compared to typical molecular complexes of low to medium molecular weights, many coordination polymers have a perceived low solubility in most conventional solvents and so are often described as “insoluble”.[17, 18, 29] In addition, it has been ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.