GEOCHEMICAL AND BIOGEOCHEMICAL
... quickly becomes a chore. After calculating a few Eh–pH diagrams, what does one learn by manually producing more plots? For many students, trees quickly come to obscure a beautiful forest. The computer can take over the mechanics of basic tasks, once they have been mastered, freeing the student to ab ...
... quickly becomes a chore. After calculating a few Eh–pH diagrams, what does one learn by manually producing more plots? For many students, trees quickly come to obscure a beautiful forest. The computer can take over the mechanics of basic tasks, once they have been mastered, freeing the student to ab ...
SCH4U TEXT BOOK
... Many terms are used inaccurately in everyday life. The word “natural” is often used in a manner suggesting that all natural compounds are safe and healthy. Similarly, the word “chemical” is commonly used to refer to artificial compounds only. The food industry uses “organic” to indicate foods that h ...
... Many terms are used inaccurately in everyday life. The word “natural” is often used in a manner suggesting that all natural compounds are safe and healthy. Similarly, the word “chemical” is commonly used to refer to artificial compounds only. The food industry uses “organic” to indicate foods that h ...
29 Sept 08 - Seattle Central
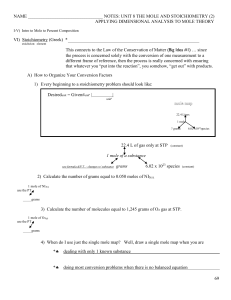
... 1. Aqueous solutions of lead(II) nitrate and sodium phosphate are mixed, resulting in the precipitate formation of lead(II) phosphate, with aqueous sodium nitrate as the other product. 2. The combustion of liquid ethanol (C2H5OH) forms carbon dioxide and water vapor. Combustion refers to the reactio ...
... 1. Aqueous solutions of lead(II) nitrate and sodium phosphate are mixed, resulting in the precipitate formation of lead(II) phosphate, with aqueous sodium nitrate as the other product. 2. The combustion of liquid ethanol (C2H5OH) forms carbon dioxide and water vapor. Combustion refers to the reactio ...
master ap chemistry - NelnetSolutions.com
... ALL RIGHTS RESERVED. No part of this work covered by the copyright herein may be reproduced or used in any form or by any means—graphic, electronic, or mechanical, including photocopying, recording, taping, Web distribution, or information storage and retrieval systems—without the prior written perm ...
... ALL RIGHTS RESERVED. No part of this work covered by the copyright herein may be reproduced or used in any form or by any means—graphic, electronic, or mechanical, including photocopying, recording, taping, Web distribution, or information storage and retrieval systems—without the prior written perm ...
Kinetics and Reaction Pathways for Propane Dehydrogenation and
... Co/H-ZSM5 catalyzes propane dehydrogenation and aromatization reactions. Initial product selectivities, product site-yields, and the 13C content and distribution in the products of 2-13C-propane show that propane undergoes two primary reactionssdehydrogenation to propene and H2 and cracking to metha ...
... Co/H-ZSM5 catalyzes propane dehydrogenation and aromatization reactions. Initial product selectivities, product site-yields, and the 13C content and distribution in the products of 2-13C-propane show that propane undergoes two primary reactionssdehydrogenation to propene and H2 and cracking to metha ...
faculty of sciences - Guru Nanak Dev University
... B.Sc. (Hons. School) Chemistry (Semester-I) (Under Credit Based Continuous Evaluation Grading System) 5. Alkenes, Cycloalkenes, Dienes and Alkynes 10 Hrs Nomenclature of alkenes, methods of formation, mechanisms of dehydration of alcohols and dehydrohalogenation of alkyl halides, regioselectivity in ...
... B.Sc. (Hons. School) Chemistry (Semester-I) (Under Credit Based Continuous Evaluation Grading System) 5. Alkenes, Cycloalkenes, Dienes and Alkynes 10 Hrs Nomenclature of alkenes, methods of formation, mechanisms of dehydration of alcohols and dehydrohalogenation of alkyl halides, regioselectivity in ...
Chemistry Exemplar Problems
... The Department of Education in Science and Mathematics (DESM), National Council of Educational Research and Training (NCERT), initiated the programme for the development of ‘Exemplar Problems’ in science and mathematics for secondary and higher secondary stages based on the subject textbooks develop ...
... The Department of Education in Science and Mathematics (DESM), National Council of Educational Research and Training (NCERT), initiated the programme for the development of ‘Exemplar Problems’ in science and mathematics for secondary and higher secondary stages based on the subject textbooks develop ...
Thomson Cathode Ray Tube Experiment (new
... Experiment. Another particle ejected during nuclear decay is the alpha particle. An alpha particle is a helium nucleus, or a helium atom without its two electrons. Consequently, an alpha particle is positively charged. Ernest Rutherford used alpha particles in his Gold Foil Experiment. 1. Start Virt ...
... Experiment. Another particle ejected during nuclear decay is the alpha particle. An alpha particle is a helium nucleus, or a helium atom without its two electrons. Consequently, an alpha particle is positively charged. Ernest Rutherford used alpha particles in his Gold Foil Experiment. 1. Start Virt ...
INFLUENCES OF THE MOLE RATIO SnO2/NaOH ON THE
... be dissolved in NaOH solution and form Sn(OH)62- anion. Sn(OH)4 + 2OH- → Sn(OH)62The possible formation process for SnO2 nanorods under hydrothermal condition can be represented in figure 6. The surfactant CTAB is a key in determining the morphology of the products. Because of the existence of surfa ...
... be dissolved in NaOH solution and form Sn(OH)62- anion. Sn(OH)4 + 2OH- → Sn(OH)62The possible formation process for SnO2 nanorods under hydrothermal condition can be represented in figure 6. The surfactant CTAB is a key in determining the morphology of the products. Because of the existence of surfa ...
Phase behavior of clathrate hydrates: a model for single and
... locations for sI guests were obtained from X-ray di)raction measurements of methane (Gutt et al., 2000), carbon dioxide (Ikeda et al., 2000), and ethylene oxide (McMullen & Je)rey, 1965). For the sI nitrogen + hydrogen hydrates, X-ray di)raction results for the methane hydrate were used to approxima ...
... locations for sI guests were obtained from X-ray di)raction measurements of methane (Gutt et al., 2000), carbon dioxide (Ikeda et al., 2000), and ethylene oxide (McMullen & Je)rey, 1965). For the sI nitrogen + hydrogen hydrates, X-ray di)raction results for the methane hydrate were used to approxima ...
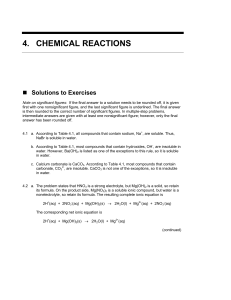
4. chemical reactions
... Some electrolyte solutions are strongly conducting because they are almost completely ionized and others are weakly conducting because they are weakly ionized. The former solutions will have many more ions to conduct electricity than will the latter solutions if both are present at the same concentr ...
... Some electrolyte solutions are strongly conducting because they are almost completely ionized and others are weakly conducting because they are weakly ionized. The former solutions will have many more ions to conduct electricity than will the latter solutions if both are present at the same concentr ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.