L-12 Spontaneity of chemical reactions
... spontaneously. The mixing of gases continues until the partial pressure of each gas becomes equal to 0.5 bar in each bulb i.e., the equilibrium is attained. We know from experience that the process cannot be reversed spontaneously - the gases do not unmix. What is the driving force behind this proce ...
... spontaneously. The mixing of gases continues until the partial pressure of each gas becomes equal to 0.5 bar in each bulb i.e., the equilibrium is attained. We know from experience that the process cannot be reversed spontaneously - the gases do not unmix. What is the driving force behind this proce ...
Stoichiometry - VernonScienceLSA
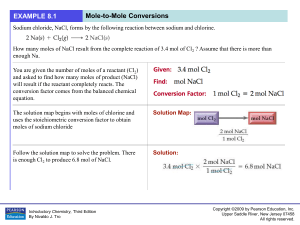
... Stoichiometry calculations allow us to find out how much of chemical #1 is involved in a chemical reaction based on the amount of chemical #2 involved. A typical problem might be “How many grams of chemical #1 must be reacted to produce 25.0 g of chemical #2?” or “What volume of chemical #1 at STP w ...
... Stoichiometry calculations allow us to find out how much of chemical #1 is involved in a chemical reaction based on the amount of chemical #2 involved. A typical problem might be “How many grams of chemical #1 must be reacted to produce 25.0 g of chemical #2?” or “What volume of chemical #1 at STP w ...
Notes - Text
... • At room temperature, an equilibrium mixture (light purple) is placed in a beaker of warm water. • The mixture turns deep blue. • This indicates a shift toward products (blue CoCl42–). • This reaction is endothermic. • For an endothermic reaction (H > 0), heat can be considered as a reactant. • Th ...
... • At room temperature, an equilibrium mixture (light purple) is placed in a beaker of warm water. • The mixture turns deep blue. • This indicates a shift toward products (blue CoCl42–). • This reaction is endothermic. • For an endothermic reaction (H > 0), heat can be considered as a reactant. • Th ...
Chemistry 2008 Multiple Choice
... [OH-] = ½(0.002 M) = 0.001 M pOH = -log(1 x 10-3) = 3 pH = 14 – 3 = 11 At the same temperature both gases have the same kinetic energy (K = 3/2RT). Amino acids: NH2–C(R)H–COOH (I hope you remember your biology). CO32- + 2 H+ CO2(g) + H2O Zn + 2 H+ H2(g) + Zn2+ Ba2+ + SO42- BaSO4(s) ...
... [OH-] = ½(0.002 M) = 0.001 M pOH = -log(1 x 10-3) = 3 pH = 14 – 3 = 11 At the same temperature both gases have the same kinetic energy (K = 3/2RT). Amino acids: NH2–C(R)H–COOH (I hope you remember your biology). CO32- + 2 H+ CO2(g) + H2O Zn + 2 H+ H2(g) + Zn2+ Ba2+ + SO42- BaSO4(s) ...
Equilibrium Part 2
... The Le Chatelier’s principle will allow us to study those factors which control the equilibrium position of a system. Henri Louis Le Chatelier (1850 - 1936) was a French chemist and a mining engineer. In 1884 Le Chatelier proposed the Law of Mobile Equilibrium, more commonly called Le Chatelier's Pr ...
... The Le Chatelier’s principle will allow us to study those factors which control the equilibrium position of a system. Henri Louis Le Chatelier (1850 - 1936) was a French chemist and a mining engineer. In 1884 Le Chatelier proposed the Law of Mobile Equilibrium, more commonly called Le Chatelier's Pr ...
Preparatory Problems of the 40th IChO - IChO-2016
... Select the redox processes from the reactions. ...
... Select the redox processes from the reactions. ...
Solved Examples
... In photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H12O6) according to the following reaction. How many grams of glucose can be synthesized from 58.5 g of CO2 ? Assume that there is more than enough water present to react with all of the CO2 . Set up the problem in the norm ...
... In photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H12O6) according to the following reaction. How many grams of glucose can be synthesized from 58.5 g of CO2 ? Assume that there is more than enough water present to react with all of the CO2 . Set up the problem in the norm ...
Oxidation-Reduction Reactions
... Na2SO3 is neutral and has no overall charge. Therefore, the sum of the charges on each atom in the compound must equal zero. We will start by identifying the overall charge on oxygen since we know that it will generally always be -2; there are 3 of them so the overall charge for oxygen is -6 (-2 x 3 ...
... Na2SO3 is neutral and has no overall charge. Therefore, the sum of the charges on each atom in the compound must equal zero. We will start by identifying the overall charge on oxygen since we know that it will generally always be -2; there are 3 of them so the overall charge for oxygen is -6 (-2 x 3 ...
CHAPTER 2 ATOMS, MOLECULES, AND IONS Questions
... b. All atoms of hydrogen have 1 proton in the nucleus. Different isotopes of hydrogen have 0, 1, or 2 neutrons in the nucleus. Because we are talking about atoms, this implies a neutral charge, which dictates 1 electron present for all hydrogen atoms. If charged ions were included, then different io ...
... b. All atoms of hydrogen have 1 proton in the nucleus. Different isotopes of hydrogen have 0, 1, or 2 neutrons in the nucleus. Because we are talking about atoms, this implies a neutral charge, which dictates 1 electron present for all hydrogen atoms. If charged ions were included, then different io ...
Equilibrium
... Chemical equilibrium can be attained whether the reaction begins with all reactants and no products, all products and no reactants, or some of both. Illustrated in the Figure 1.2 are the changes in the concentrations of H2 , I2 , and HI for two different initial reaction mixtures. In the situation d ...
... Chemical equilibrium can be attained whether the reaction begins with all reactants and no products, all products and no reactants, or some of both. Illustrated in the Figure 1.2 are the changes in the concentrations of H2 , I2 , and HI for two different initial reaction mixtures. In the situation d ...
Chapter 4: Oxidation and Reduction MH5 4
... Unit 3 Oxidation and Reduction Chemistry 020, R. R. Martin 1 Introduction Another important type of reaction in aqueous solution involves the transfer of electrons between two species. This is called an oxidation-reduction or a redox reaction. What happens when zinc pellets are added to an acid? The ...
... Unit 3 Oxidation and Reduction Chemistry 020, R. R. Martin 1 Introduction Another important type of reaction in aqueous solution involves the transfer of electrons between two species. This is called an oxidation-reduction or a redox reaction. What happens when zinc pellets are added to an acid? The ...
EDTA Titrations
... buffer the pH to (a) 10.0. What is aY4- ? (b) What is aY4- if the pH of the solution is buffered to 11.0? ...
... buffer the pH to (a) 10.0. What is aY4- ? (b) What is aY4- if the pH of the solution is buffered to 11.0? ...
Mole and Energy - Deans Community High School
... Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change in enthalpy, the ...
... Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change in enthalpy, the ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.