National German Competition
... 4. The radiation of 1 is executed in a closed vessel. After the reaction there is an overpressure within the vessel, a gas was generated. 5. The reaction was followed by infrared spectroscopy. A sample of compound 1 gave a certain spectrum (upper part of the image). Then the sample was radiated with ...
... 4. The radiation of 1 is executed in a closed vessel. After the reaction there is an overpressure within the vessel, a gas was generated. 5. The reaction was followed by infrared spectroscopy. A sample of compound 1 gave a certain spectrum (upper part of the image). Then the sample was radiated with ...
Electrochemistry
... lectrochemistry constitutes one of the most important interfaces between chemistry and everyday life. Every time you start your car, turn on your calculator, use your smartphone, or listen to a radio at the beach, you are depending on electrochemical reactions. Our society sometimes seems to run alm ...
... lectrochemistry constitutes one of the most important interfaces between chemistry and everyday life. Every time you start your car, turn on your calculator, use your smartphone, or listen to a radio at the beach, you are depending on electrochemical reactions. Our society sometimes seems to run alm ...
chemistry - Rwanda Education Board
... Evaluation or assessment has to be planned and carried out as a part of the classroom activities. Different methods of assessment can be conducted. These may be in the form of assignments, oral questioning and answering, observations and interviews. Based on the given responses, teacher can rectify ...
... Evaluation or assessment has to be planned and carried out as a part of the classroom activities. Different methods of assessment can be conducted. These may be in the form of assignments, oral questioning and answering, observations and interviews. Based on the given responses, teacher can rectify ...
Hardness - ChemGod.com
... Determining Hardness If you are looking for “hardness”, what are you actually ...
... Determining Hardness If you are looking for “hardness”, what are you actually ...
Chapter 13 PowerPoint
... Depicting Equilibrium Since, in a system at equilibrium, both the forward and reverse reactions are being carried out, we write its equation with a double arrow. ...
... Depicting Equilibrium Since, in a system at equilibrium, both the forward and reverse reactions are being carried out, we write its equation with a double arrow. ...
Study Material - Class- XI- Chemistry
... The smallest particle of an element, which may or may not have independent existence, is called an atom, while the smallest particle of a substance which is capable of independent existence is called a molecule. Molecules are classified as homoatomic and heteroatomic. Homoatomic molecules are made u ...
... The smallest particle of an element, which may or may not have independent existence, is called an atom, while the smallest particle of a substance which is capable of independent existence is called a molecule. Molecules are classified as homoatomic and heteroatomic. Homoatomic molecules are made u ...
Regents Chemistry - New York Science Teacher
... 4NH3 + 5O2 -> 4NO + 6H2O What is the minimum number of moles of O2 that are needed to completely react 1ith 16 moles of NH3? ...
... 4NH3 + 5O2 -> 4NO + 6H2O What is the minimum number of moles of O2 that are needed to completely react 1ith 16 moles of NH3? ...
Sample pages 2 PDF
... Chemical kinetics is the branch of chemistry that measures rates of chemical reactions, studies the factors that influence them, designs and prepares new catalysts, and interprets the results at the molecular level. The independent variable of chemical kinetics, from the chemical reaction starting m ...
... Chemical kinetics is the branch of chemistry that measures rates of chemical reactions, studies the factors that influence them, designs and prepares new catalysts, and interprets the results at the molecular level. The independent variable of chemical kinetics, from the chemical reaction starting m ...
orange review book_2014_key
... 18. Which substance can be broken down by a chemical change? (1) antimony (3) hexane (2) carbon (4) sulfur 19. One similarity between all mixtures and compounds is that both (1) are heterogeneous (2) consist of two or more substances (3) are homogeneous (4) are heterogeneous 20. A dilute ...
... 18. Which substance can be broken down by a chemical change? (1) antimony (3) hexane (2) carbon (4) sulfur 19. One similarity between all mixtures and compounds is that both (1) are heterogeneous (2) consist of two or more substances (3) are homogeneous (4) are heterogeneous 20. A dilute ...
Effects of Neutral Salts of the Lyotropic Series on the Physical
... decreasing extensigraph length values (Fig. 2). At low concentrations the nonchaotropic salts (sodium chloride and sodium bromide) increased extensigraph length, with small reductions occurring at higher concentrations. With sodium chlorate, low concentrations caused increases in curve length and at ...
... decreasing extensigraph length values (Fig. 2). At low concentrations the nonchaotropic salts (sodium chloride and sodium bromide) increased extensigraph length, with small reductions occurring at higher concentrations. With sodium chlorate, low concentrations caused increases in curve length and at ...
Lecture 11 Notes
... Intermolecular forces explain many properties of liquids. (a) Molecules at the surface interact with other surface molecules and with molecules directly below the surface. (b) Molecules in the ...
... Intermolecular forces explain many properties of liquids. (a) Molecules at the surface interact with other surface molecules and with molecules directly below the surface. (b) Molecules in the ...
Stoichiometry - HCC Learning Web
... Practice makes perfect! Working stoichiometry problems properly will strengthen your skills in working many other types of chemistry problems as well. ...
... Practice makes perfect! Working stoichiometry problems properly will strengthen your skills in working many other types of chemistry problems as well. ...
ChemQuest 1 Information: Qualitative vs. Quantitative Critical
... 6. Is it possible to answer this question: How many mg are in one km? Explain. No, because milligrams and kilometers are different units of measure. ...
... 6. Is it possible to answer this question: How many mg are in one km? Explain. No, because milligrams and kilometers are different units of measure. ...
Solution - gearju.com
... The molecular formula might be the same as the empirical formula or some integral multiple of it (for example, two, three, four, or more times the empirical formula). Comparing the ratio of the molar mass to the molar mass of the empirical formula will show the integral relationship between the empi ...
... The molecular formula might be the same as the empirical formula or some integral multiple of it (for example, two, three, four, or more times the empirical formula). Comparing the ratio of the molar mass to the molar mass of the empirical formula will show the integral relationship between the empi ...
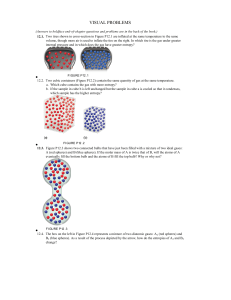
visual problems - Western Oregon University
... a. Use the appropriate ∆G°f value(s) from Appendix 4 to calculate ∆G°rxn for the reaction known as steam–methane reforming: CH4(g) + H2O(g) → CO(g) + 3 H2(g) b. To drive this nonspontaneous reaction the CO that is produced can be oxidized to CO 2 using more steam: CO(g) + H2O(g) → CO2(g) + H2(g) Us ...
... a. Use the appropriate ∆G°f value(s) from Appendix 4 to calculate ∆G°rxn for the reaction known as steam–methane reforming: CH4(g) + H2O(g) → CO(g) + 3 H2(g) b. To drive this nonspontaneous reaction the CO that is produced can be oxidized to CO 2 using more steam: CO(g) + H2O(g) → CO2(g) + H2(g) Us ...
NCERT Solution - Mywayteaching
... causes acid rain. Acid rain damages soil, plants, and buildings, especially those made of marble. ...
... causes acid rain. Acid rain damages soil, plants, and buildings, especially those made of marble. ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.