CHAPTER -IX Apparent Molar Volumes and Viscosity
... mixtures increases. Since SV* is a measure of ion–ion interactions, the results indicate the presence of weak ion–ion interactions in the solutions at all experimental temperatures, and these interactions further decrease with a rise in concentration of 2-methoxy ethanol in the mixtures. In other wo ...
... mixtures increases. Since SV* is a measure of ion–ion interactions, the results indicate the presence of weak ion–ion interactions in the solutions at all experimental temperatures, and these interactions further decrease with a rise in concentration of 2-methoxy ethanol in the mixtures. In other wo ...
Hydrogen dissociation under equilibrium and non
... Computer simulations are a useful tool for a realistic study of systems under conditions that are not easy to achieve in a laboratory, such as high temperature, pressure, concentration and other extreme conditions. Quantum mechanics is one option, but as these simulations becomes very time consuming ...
... Computer simulations are a useful tool for a realistic study of systems under conditions that are not easy to achieve in a laboratory, such as high temperature, pressure, concentration and other extreme conditions. Quantum mechanics is one option, but as these simulations becomes very time consuming ...
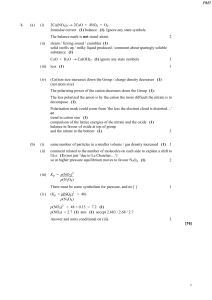
1. (a) (i) 2Ca(NO3)2 → 2CaO + 4NO2 + O2 formulae correct (1
... • That each molecule can form more than one hydrogen bond because of the two OH (and two S=O groups) / or a description of hydrogen bonds in this case / or a diagram showing the hydrogen bonds (1) ...
... • That each molecule can form more than one hydrogen bond because of the two OH (and two S=O groups) / or a description of hydrogen bonds in this case / or a diagram showing the hydrogen bonds (1) ...
AS Chemistry 1
... We can picture the hydrogen atom — the simplest of all atoms with one electron and one proton in the nucleus — by considering a pea placed in the centre of a football pitch, to represent the nucleus with its proton. On this scale the electron will revolve in a circular orbit round the goalposts. Bet ...
... We can picture the hydrogen atom — the simplest of all atoms with one electron and one proton in the nucleus — by considering a pea placed in the centre of a football pitch, to represent the nucleus with its proton. On this scale the electron will revolve in a circular orbit round the goalposts. Bet ...
Major 01 - KFUPM Faculty List
... C. When placed in water a gas is formed. Again, formation of a gas indicates a chemical reaction. D. When placed in contact with chlorine it forms a compound that melts at 801oC. Formation of a compound with a higher melting point than sodium indicates a chemical change. E. In contact with the skin, ...
... C. When placed in water a gas is formed. Again, formation of a gas indicates a chemical reaction. D. When placed in contact with chlorine it forms a compound that melts at 801oC. Formation of a compound with a higher melting point than sodium indicates a chemical change. E. In contact with the skin, ...
Chemistry 121: Topic 2 - From Atoms to Stoichiometry Topic 2
... 12) A solution containing 72.7 mL of liquid A and 59.4 mL of liquid B, has a density of 1.024 g/mL. On adding a further 60.0 mL of liquid B to the mixture, the density increases to 1.1301 g/mL. Assuming that volumes are additive, calculate the densities of both liquids. Initially, we have (know); ¾ ...
... 12) A solution containing 72.7 mL of liquid A and 59.4 mL of liquid B, has a density of 1.024 g/mL. On adding a further 60.0 mL of liquid B to the mixture, the density increases to 1.1301 g/mL. Assuming that volumes are additive, calculate the densities of both liquids. Initially, we have (know); ¾ ...
Kinetics of Oxidation of Benzyl Alcohol with Dilute Nitric Acid
... Table 2 shows that the ratio of alcohol consumed to HNO3 at different reaction conditions is ∼2.0. This is consistent with the stoichiometric requirement indicated by eq 7. In addition, the gas-phase analysis (Suchak et al., 1989) was performed in an operating plant, using the HNO3 oxidation of benz ...
... Table 2 shows that the ratio of alcohol consumed to HNO3 at different reaction conditions is ∼2.0. This is consistent with the stoichiometric requirement indicated by eq 7. In addition, the gas-phase analysis (Suchak et al., 1989) was performed in an operating plant, using the HNO3 oxidation of benz ...
CHEMICAL EQUILIBRIUM (Part II)II)
... Constants Knowing the equilibrium constant (Kc or ) and/or the initial concentrations of KP) and/or the initial concentrations of reactants and products for a given reaction allows you to predict several features of that reaction, such as: o whether the reaction tends to occur or not. or not ...
... Constants Knowing the equilibrium constant (Kc or ) and/or the initial concentrations of KP) and/or the initial concentrations of reactants and products for a given reaction allows you to predict several features of that reaction, such as: o whether the reaction tends to occur or not. or not ...
Organizing Topic - Staunton City Schools
... retrieve enough of each substance to be massed. The mystery substance should be an insoluble substance, such as small plastic pellets. Students will be able to separate the various materials by different densities. Procedure ...
... retrieve enough of each substance to be massed. The mystery substance should be an insoluble substance, such as small plastic pellets. Students will be able to separate the various materials by different densities. Procedure ...
Support Material
... Explain how vacancies are introduced in a solid NaCl crystal when divalent cations (M2+) are added to molten NaCl. ...
... Explain how vacancies are introduced in a solid NaCl crystal when divalent cations (M2+) are added to molten NaCl. ...
Holt Modern Chemistry Workbook
... living things are molecules. A molecule is a neutral group of atoms that are held together by covalent bonds. A molecule formed by polar-covalent bonds is still considered neutral because the sum of the partial charges on either end of the bond is zero. A molecular compound is any chemical compound ...
... living things are molecules. A molecule is a neutral group of atoms that are held together by covalent bonds. A molecule formed by polar-covalent bonds is still considered neutral because the sum of the partial charges on either end of the bond is zero. A molecular compound is any chemical compound ...
Laboratory Manual
... Playing a game is more enjoyable than just watching or reading about it. This is also true about learning chemistry. Getting to actually do experiments is a lot of fun. But chemistry can also be dangerous, and getting hurt is definitely not fun! For this reason there are safety rules to follow and p ...
... Playing a game is more enjoyable than just watching or reading about it. This is also true about learning chemistry. Getting to actually do experiments is a lot of fun. But chemistry can also be dangerous, and getting hurt is definitely not fun! For this reason there are safety rules to follow and p ...
Gases - chemmybear.com
... (a) Reducing the temperature of a gas reduces the average kinetic energy (or velocity) of the gas molecules. This would reduce the number (or frequency) of collisions of gas molecules with the surface of the balloon; [OR decrease the momentum change that occurs when the gas molecules strike the ball ...
... (a) Reducing the temperature of a gas reduces the average kinetic energy (or velocity) of the gas molecules. This would reduce the number (or frequency) of collisions of gas molecules with the surface of the balloon; [OR decrease the momentum change that occurs when the gas molecules strike the ball ...
Mechanisms and energetics of surface reactions at the copper
... is negative on the Gibbs free energy (3, 4, 5). Since the entropy effect depends linearly on the temperature, ∆G° will eventually become negative when the temperature is increased. The entropy effect for the formation of cuprous oxide (3) is –T∆S° = –0.14 eV, meaning that ∆G = 0 when T = 1,300 K. Si ...
... is negative on the Gibbs free energy (3, 4, 5). Since the entropy effect depends linearly on the temperature, ∆G° will eventually become negative when the temperature is increased. The entropy effect for the formation of cuprous oxide (3) is –T∆S° = –0.14 eV, meaning that ∆G = 0 when T = 1,300 K. Si ...
Electrodeposition of rare earth metals in ionic liquids
... Chlonine based ionic liquids In 2012 Ishii et al. [22] investigated the physical and electrochemical properties of choline‐ based ionic liquids and their capacity to reduce iron and neodymium cations. The novel cationic blended ionic liquids which were constituted by the 2‐ h ...
... Chlonine based ionic liquids In 2012 Ishii et al. [22] investigated the physical and electrochemical properties of choline‐ based ionic liquids and their capacity to reduce iron and neodymium cations. The novel cationic blended ionic liquids which were constituted by the 2‐ h ...
chemical reaction
... represented in the equation must be adjusted so that the numbers and types of atoms are the same on both sides of the equation. • This process is called balancing an equation and is carried out by inserting coefficients. ...
... represented in the equation must be adjusted so that the numbers and types of atoms are the same on both sides of the equation. • This process is called balancing an equation and is carried out by inserting coefficients. ...
CHEM 1212 Principles of Chemistry II Course Study Guide
... on reserve at the library. ONLY use these to check problems you have already attempted. Reading the solutions will not help (See study tip #1). 3) Do not forget the “visual problems.” These are “conceptual” like your final and many test questions. 4) If you have the access code, you can also do the ...
... on reserve at the library. ONLY use these to check problems you have already attempted. Reading the solutions will not help (See study tip #1). 3) Do not forget the “visual problems.” These are “conceptual” like your final and many test questions. 4) If you have the access code, you can also do the ...
Effects of mannose, fructose, and fucose
... The tendencies of proteins to decompose denature, and aggregate are critical problems in the biotechnology, food, cosmetics, and pharmaceutical industries. The chemical (oxidation, deamidation, and hydrolysis) and physical (unfolding and aggregation) changes of proteins during the formulation proces ...
... The tendencies of proteins to decompose denature, and aggregate are critical problems in the biotechnology, food, cosmetics, and pharmaceutical industries. The chemical (oxidation, deamidation, and hydrolysis) and physical (unfolding and aggregation) changes of proteins during the formulation proces ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.