Electrochemical and Spectroelectrochemical Studies of Dyes used
... experimenting with an electrolytic cell made up of two metal electrodes placed in conducting solution. He observed that current increased when the electrolytic cell was exposed to light. At that time there was not any relevant interest in finding new sources of energy that could be environmentally f ...
... experimenting with an electrolytic cell made up of two metal electrodes placed in conducting solution. He observed that current increased when the electrolytic cell was exposed to light. At that time there was not any relevant interest in finding new sources of energy that could be environmentally f ...
Chemistry - A Quantitative Science
... atomic weight* of any atom or in one gram molecular weight of any molecule. Thus, the * A gram atomic weight is a mass of atoms equal to the atomic weight mass of a mole of any substance, which is known as its molar mass (Mm), equals its of the atom expressed in grams. atomic or molecular weight exp ...
... atomic weight* of any atom or in one gram molecular weight of any molecule. Thus, the * A gram atomic weight is a mass of atoms equal to the atomic weight mass of a mole of any substance, which is known as its molar mass (Mm), equals its of the atom expressed in grams. atomic or molecular weight exp ...
Chemistry of Riming: The Retention of Organic and Inorganic
... boxylic acids may contribute significantly to the free acidity in precipitation (Kawamura et al., 1996). Furthermore, they have a low photochemical reactivity in the atmospheric gas phase (residence time: 2 – 3 days), so that important sinks for these organic acids are dry and wet deposition (Chebbi ...
... boxylic acids may contribute significantly to the free acidity in precipitation (Kawamura et al., 1996). Furthermore, they have a low photochemical reactivity in the atmospheric gas phase (residence time: 2 – 3 days), so that important sinks for these organic acids are dry and wet deposition (Chebbi ...
4) What is the term for the procedure of collecting data and recording
... A sample of rose gold is: 12.0 grams gold, 5.0 grams silver, and 7.0 grams copper by mass. What is the percent of copper in the sample? A) 12% B) 29% C) 50% D) 58% E) 75% Stainless steel is composed of iron, manganese, chromium, and nickel. If a 2.00 g sample was analyzed and found to contain 2.75% ...
... A sample of rose gold is: 12.0 grams gold, 5.0 grams silver, and 7.0 grams copper by mass. What is the percent of copper in the sample? A) 12% B) 29% C) 50% D) 58% E) 75% Stainless steel is composed of iron, manganese, chromium, and nickel. If a 2.00 g sample was analyzed and found to contain 2.75% ...
The Equilibrium Constant K
... that for the reaction written in reverse. When the balanced equation for a reaction is multiplied by a factor of n, the equilibrium expression for the new reaction is the original expression raised to the nth power; thus Knew = (Koriginal)n. K values are usually written without units. ...
... that for the reaction written in reverse. When the balanced equation for a reaction is multiplied by a factor of n, the equilibrium expression for the new reaction is the original expression raised to the nth power; thus Knew = (Koriginal)n. K values are usually written without units. ...
Combined EPR and Molecular Modeling Study of PPI Dendrimers
... solution, and to differentiate the internal/external interacting sites of the dendrimers in respect to their availability for ion complexation and trapping.10−16 Focusing on PPI of generation n up to four (PPI-Gn, hereafter; since the PPI dendrimers are commercially available, we adopted the names of ...
... solution, and to differentiate the internal/external interacting sites of the dendrimers in respect to their availability for ion complexation and trapping.10−16 Focusing on PPI of generation n up to four (PPI-Gn, hereafter; since the PPI dendrimers are commercially available, we adopted the names of ...
ROSINA/DFMS and IES observations of 67P: Ion-neutral - HAL-Insu
... 67P (Wurz et al. 2015) is observed. When Rosetta was 10 km from the comet in mid-October 2014, DFMS observed nonvolatile refractory species (Na, K, Si, and Ca) in the coma. Observations (not shown here) of sputtered dust refractory species during the period from 30 October through 15 December 2014 d ...
... 67P (Wurz et al. 2015) is observed. When Rosetta was 10 km from the comet in mid-October 2014, DFMS observed nonvolatile refractory species (Na, K, Si, and Ca) in the coma. Observations (not shown here) of sputtered dust refractory species during the period from 30 October through 15 December 2014 d ...
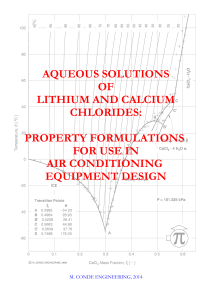
Aqueous solutions of lithium and calcium chlorides
... The R&D effort in this field has increased significatively in the last twenty years, in particular because of the perceived need to replace the more conventional technologies that use mechanical refrigeration equipment. Uncertainties regarding the availability of safe and suitable working fluids for ...
... The R&D effort in this field has increased significatively in the last twenty years, in particular because of the perceived need to replace the more conventional technologies that use mechanical refrigeration equipment. Uncertainties regarding the availability of safe and suitable working fluids for ...
Test
... After equilibrium was reached, the volume was increased to 2.0 liters, while the temperature was kept at 300 K. The result of this change was a) an increase in Kp b) a decrease in Kp c) a shift in the equilibrium position to the right d) a shift in the equilibrium position to the left e) none of the ...
... After equilibrium was reached, the volume was increased to 2.0 liters, while the temperature was kept at 300 K. The result of this change was a) an increase in Kp b) a decrease in Kp c) a shift in the equilibrium position to the right d) a shift in the equilibrium position to the left e) none of the ...
Dynamics of capillary transport in semi
... and a-L-guluronic acid (G units).35 In the presence of multivalent cations, such as Ca2+, alginate forms a gel linking only the G units and forming a network in the form of an ‘‘egg box’’model.36,37 The composition of M and G units depends, among other factors, on the plant species, season, and grow ...
... and a-L-guluronic acid (G units).35 In the presence of multivalent cations, such as Ca2+, alginate forms a gel linking only the G units and forming a network in the form of an ‘‘egg box’’model.36,37 The composition of M and G units depends, among other factors, on the plant species, season, and grow ...
The role of formic acid pretreatment in improving the carboxyl
... solution. The reaction was stopped after 6 h by adding ethanol (ca. 5 mL). The TEMPO-oxidized fiber suspension was filtered and washed thoroughly with de-ionized water on a filter cloth set in a Büchner funnel. The water-insoluble fractions thus obtained were then collected and kept at 4 ºC to preve ...
... solution. The reaction was stopped after 6 h by adding ethanol (ca. 5 mL). The TEMPO-oxidized fiber suspension was filtered and washed thoroughly with de-ionized water on a filter cloth set in a Büchner funnel. The water-insoluble fractions thus obtained were then collected and kept at 4 ºC to preve ...
Magnesium based ternary metal hydrides containing alkali and
... K. Yvon, B. Bertheville / Journal of Alloys and Compounds xxx (2006) xxx–xxx ...
... K. Yvon, B. Bertheville / Journal of Alloys and Compounds xxx (2006) xxx–xxx ...
mass
... 6.022X1023 methane molecules and 6.022X1023 water molecules 6.022X1023 to 6.022X1023 is also a 1 to 1 ratio between CH4 and H2O Realizing that 6.022X1023 molecules is also 1 mole leads to the idea that 1 mole methane to 1 mole water is also a 1 to 1 ratio. 0.5 mole to 0.5 mole is also a 1 to 1 ratio ...
... 6.022X1023 methane molecules and 6.022X1023 water molecules 6.022X1023 to 6.022X1023 is also a 1 to 1 ratio between CH4 and H2O Realizing that 6.022X1023 molecules is also 1 mole leads to the idea that 1 mole methane to 1 mole water is also a 1 to 1 ratio. 0.5 mole to 0.5 mole is also a 1 to 1 ratio ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... it also determines the amounts of the other ingredients we will use! ...
... it also determines the amounts of the other ingredients we will use! ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.