- Kendriya Vidyalaya No. 2 Raipur
... Temperature--There are three common scales to measure temperature—°C (degreecelsius), °F (degree Fahrenheit) and K (kelvin). Here, K is the SI unit. K = °C + 273.15 Note—Temperature below 0 °C (i.e. negative values) are possible in Celsius scale but in Kelvin scale, negative temperature is not possi ...
... Temperature--There are three common scales to measure temperature—°C (degreecelsius), °F (degree Fahrenheit) and K (kelvin). Here, K is the SI unit. K = °C + 273.15 Note—Temperature below 0 °C (i.e. negative values) are possible in Celsius scale but in Kelvin scale, negative temperature is not possi ...
Fe(III) (Oxy)hydroxide Compositional Series
... 2.1. Synthesis of CrxFe1-x-(oxy)hydroxides. Many different synthesis methods have been used to produce the CrxFe1-x(oxy)hydroxide, most commonly by reduction of Cr(VI) by Fe(II)-containing compounds or by hydrolysis of Cr(III)- and Fe(III)-containing solutions using different alkaline sources. To pr ...
... 2.1. Synthesis of CrxFe1-x-(oxy)hydroxides. Many different synthesis methods have been used to produce the CrxFe1-x(oxy)hydroxide, most commonly by reduction of Cr(VI) by Fe(II)-containing compounds or by hydrolysis of Cr(III)- and Fe(III)-containing solutions using different alkaline sources. To pr ...
Polymer Electrolytes
... • cations in liquids or low MW polymer; ions can move together with their coordinated solvent, not in high MW polymer • When MW > 3200 g/mol, MW of polymer has no significant effect on cation mobility. ...
... • cations in liquids or low MW polymer; ions can move together with their coordinated solvent, not in high MW polymer • When MW > 3200 g/mol, MW of polymer has no significant effect on cation mobility. ...
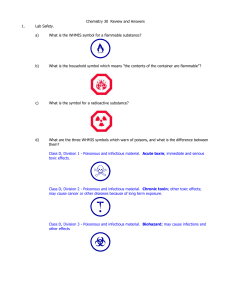
REVIEW and answers
... If 0.100 mol of HI is placed in a 1.00 L container and allowed to reach equilibrium, find the concentration of each species at equilibrium. use the ICE box to solve the problem, where the concentration of H 2 and I2 are set to x and the concentration of HI is (0.100 mol/L - 2x). Solve for x to get t ...
... If 0.100 mol of HI is placed in a 1.00 L container and allowed to reach equilibrium, find the concentration of each species at equilibrium. use the ICE box to solve the problem, where the concentration of H 2 and I2 are set to x and the concentration of HI is (0.100 mol/L - 2x). Solve for x to get t ...
Chapter -
... – Mole ratio (divide all moles by the smallest number of moles) – The numbers represent subscripts. • If the numbers are not whole numbers, multiply by some factor to make them whole. ...
... – Mole ratio (divide all moles by the smallest number of moles) – The numbers represent subscripts. • If the numbers are not whole numbers, multiply by some factor to make them whole. ...
Introduction to Inorganic Chemistry
... Chemistry comprises two related but distinct activities: (i) the quest for an understanding of matter and material change, (ii) the utilization of material change for human ends. Ideally, the first activity provides the necessary know-how for the pursuit of the second, but in practice, the help it c ...
... Chemistry comprises two related but distinct activities: (i) the quest for an understanding of matter and material change, (ii) the utilization of material change for human ends. Ideally, the first activity provides the necessary know-how for the pursuit of the second, but in practice, the help it c ...
aq - Valencia College
... i. This can be added to the graph by right-clicking any point and selecting the “Add Trendline” option in Excel. e. After the best-fit straight line is produced, an equation for the line will be reported on the graph using the following generic formula: y = mx + b. f. The density of the material is ...
... i. This can be added to the graph by right-clicking any point and selecting the “Add Trendline” option in Excel. e. After the best-fit straight line is produced, an equation for the line will be reported on the graph using the following generic formula: y = mx + b. f. The density of the material is ...
Downloaded - Maynooth University ePrints and eTheses Archive
... it was decide to try to isolate the mercury(II) complexes under different conditions to see if a number of species existed in solution. Varying the time of reaction, temperature or solvent did not result in complexes of different conformation or symmetries but did give rise to an unusual outcome. Th ...
... it was decide to try to isolate the mercury(II) complexes under different conditions to see if a number of species existed in solution. Varying the time of reaction, temperature or solvent did not result in complexes of different conformation or symmetries but did give rise to an unusual outcome. Th ...
TERMS AND DEFINITIONS IN THERMOCHEMISTRY
... also be produced indirectly if the copper is first dissolved in nitric acid to form copper(II) nitrate, and this is then heated to form the copper(II) oxide. In either case the total enthalpy change is the same. Hess's law allows the calculation of enthalpy changes which are difficult or impossible ...
... also be produced indirectly if the copper is first dissolved in nitric acid to form copper(II) nitrate, and this is then heated to form the copper(II) oxide. In either case the total enthalpy change is the same. Hess's law allows the calculation of enthalpy changes which are difficult or impossible ...
431 KB / 47 pages
... (a) We have seen (Investigate This 10.2) that electrolysis of a dilute aqueous solution of an ionic compound (magnesium sulfate) produces a gas at both electrodes and a basic solution at the cathode and acidic solution at the anode, just as the problem statement says is observed here for a dilute aq ...
... (a) We have seen (Investigate This 10.2) that electrolysis of a dilute aqueous solution of an ionic compound (magnesium sulfate) produces a gas at both electrodes and a basic solution at the cathode and acidic solution at the anode, just as the problem statement says is observed here for a dilute aq ...
chem textbook 2015 - Manitowoc Public School District
... The intent of the following information is to give answers and suggestions to questions that students often ask, it is meant to work in conjunction with Suggestions for Boosting Grades. “It makes sense in class but not when I get home.” This generally means that your notes are incomplete, meaning th ...
... The intent of the following information is to give answers and suggestions to questions that students often ask, it is meant to work in conjunction with Suggestions for Boosting Grades. “It makes sense in class but not when I get home.” This generally means that your notes are incomplete, meaning th ...
entropy - KFUPM Faculty List
... The standard free energy of reaction (G°rxn) is the freeenergy change for a reaction when it occurs under standardstate conditions—that is, when reactants in their standard states are converted to products in their standard states. ...
... The standard free energy of reaction (G°rxn) is the freeenergy change for a reaction when it occurs under standardstate conditions—that is, when reactants in their standard states are converted to products in their standard states. ...
paper - General Atomics Fusion Group
... transportation fuel that has the potential to displace fossil fuels. Hydrogen will be particularly advantageous when coupled with fuel cells. Fuel cells have higher efficiency than conventional battery/internal combustion engine combinations and do not produce nitrogen oxides during low-temperature ...
... transportation fuel that has the potential to displace fossil fuels. Hydrogen will be particularly advantageous when coupled with fuel cells. Fuel cells have higher efficiency than conventional battery/internal combustion engine combinations and do not produce nitrogen oxides during low-temperature ...
- StarBooks
... three significant figures, while 16.00has four significant figures. Zeros at the end of a number without decimal point are ambiguous. In exponential notations, the numerical portion represents the number of significant figures. For example, 0.00045 isexpressed as 4.5 x 10 -4 in terms of scientific n ...
... three significant figures, while 16.00has four significant figures. Zeros at the end of a number without decimal point are ambiguous. In exponential notations, the numerical portion represents the number of significant figures. For example, 0.00045 isexpressed as 4.5 x 10 -4 in terms of scientific n ...
Chemistry
... subject or as part of a balanced science course. This syllabus is designed to place less emphasis on factual material and greater emphasis on the understanding and application of scientific concepts and principles. This approach has been adopted in recognition of the need for students to develop ski ...
... subject or as part of a balanced science course. This syllabus is designed to place less emphasis on factual material and greater emphasis on the understanding and application of scientific concepts and principles. This approach has been adopted in recognition of the need for students to develop ski ...
Electrochemistry
... lectrochemistry constitutes one of the most important interfaces between chemistry and everyday life. Every time you start your car, turn on your calculator, use your smartphone, or listen to a radio at the beach, you are depending on electrochemical reactions. Our society sometimes seems to run alm ...
... lectrochemistry constitutes one of the most important interfaces between chemistry and everyday life. Every time you start your car, turn on your calculator, use your smartphone, or listen to a radio at the beach, you are depending on electrochemical reactions. Our society sometimes seems to run alm ...
National German Competition
... 4. The radiation of 1 is executed in a closed vessel. After the reaction there is an overpressure within the vessel, a gas was generated. 5. The reaction was followed by infrared spectroscopy. A sample of compound 1 gave a certain spectrum (upper part of the image). Then the sample was radiated with ...
... 4. The radiation of 1 is executed in a closed vessel. After the reaction there is an overpressure within the vessel, a gas was generated. 5. The reaction was followed by infrared spectroscopy. A sample of compound 1 gave a certain spectrum (upper part of the image). Then the sample was radiated with ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.