Cliffs Notes
... Technical Editor: Christopher Bushee Production Proofreader: Joel K. Draper Hungry Minds Indianapolis Production Services ...
... Technical Editor: Christopher Bushee Production Proofreader: Joel K. Draper Hungry Minds Indianapolis Production Services ...
Proton Chemical Shift Tensors and Hydrogen Bond Geometry: A 1H
... to obtain 1H CS tensors for the stationary water molecules, since it is the stationary, rather than the motionally aVeraged CS tensor, that is directly related to the chemical environment. There are two possible ways of obtaining this information. The direct approach is to stop the flipping motion b ...
... to obtain 1H CS tensors for the stationary water molecules, since it is the stationary, rather than the motionally aVeraged CS tensor, that is directly related to the chemical environment. There are two possible ways of obtaining this information. The direct approach is to stop the flipping motion b ...
Final Exam Review Notes
... Some numbers are very large or very small difficult to express. For example, Avogadro’s number = 602,000,000,000,000,000,000,000 an electron’s mass = 0.000 000 000 000 000 000 000 000 000 91 kg Also, it's not clear how many sig figs there are in some measurements. For example, Express 100.0 g to 3 ...
... Some numbers are very large or very small difficult to express. For example, Avogadro’s number = 602,000,000,000,000,000,000,000 an electron’s mass = 0.000 000 000 000 000 000 000 000 000 91 kg Also, it's not clear how many sig figs there are in some measurements. For example, Express 100.0 g to 3 ...
BARIUM NITRATE
... Precipitated BaSO4 is often used in many industrial applications. Blanc fixe and Lithopone are made by the reactions of barium sulfide with sodium sulfate and zinc sulfate, respectively. Reactions Barium sulfate is one of the most insoluble salts of barium. It does not undergo double decomposition r ...
... Precipitated BaSO4 is often used in many industrial applications. Blanc fixe and Lithopone are made by the reactions of barium sulfide with sodium sulfate and zinc sulfate, respectively. Reactions Barium sulfate is one of the most insoluble salts of barium. It does not undergo double decomposition r ...
On the formation of radiation fogs under heavily polluted
... Our future goal is to model observed pollution fogs; however, that will require more sophisticated models than those used in the present study. For example, when modeling the London type smogs, it would be highly desirable to describe the condensation of hydrochloric acid. However, the thermodynamic ...
... Our future goal is to model observed pollution fogs; however, that will require more sophisticated models than those used in the present study. For example, when modeling the London type smogs, it would be highly desirable to describe the condensation of hydrochloric acid. However, the thermodynamic ...
Ch 3
... oxygen atom, the molar mass is 72.06 + 12.10 + 96.00 = 180.16 g/mol So to calculate the mass percent of carbon in glucose, we just use the mass of carbon in one mole of glucose over the mass of one mole of glucose to get the mass fraction and then multiply by 100. Mass % C = (76.06g/mol / 180.16 g/m ...
... oxygen atom, the molar mass is 72.06 + 12.10 + 96.00 = 180.16 g/mol So to calculate the mass percent of carbon in glucose, we just use the mass of carbon in one mole of glucose over the mass of one mole of glucose to get the mass fraction and then multiply by 100. Mass % C = (76.06g/mol / 180.16 g/m ...
8 Chemical Equations Chapter Outline Chemical Equations
... Write the balanced chemical equation for the reaction of magnesium hydroxide and phosphoric acid to form magnesium phosphate and water. a. 3 Mg(OH)2 + 2 H3PO4 b. Mg(OH)2 + H3PO4 ...
... Write the balanced chemical equation for the reaction of magnesium hydroxide and phosphoric acid to form magnesium phosphate and water. a. 3 Mg(OH)2 + 2 H3PO4 b. Mg(OH)2 + H3PO4 ...
Exam Review
... Petroleum is a complex mixture of hundreds of thousands of compounds. Some of these compounds boil at temperatures as low as 20ºC. The least volatile compounds of crude oil, boil at temperatures above 400ºC. The differences in boiling points of the compounds making up petroleum enable the separation ...
... Petroleum is a complex mixture of hundreds of thousands of compounds. Some of these compounds boil at temperatures as low as 20ºC. The least volatile compounds of crude oil, boil at temperatures above 400ºC. The differences in boiling points of the compounds making up petroleum enable the separation ...
List of Papers - Research Portal
... Cell-based sensors have used living organisms such as bacteria, yeast, algae, fungi, animals and plants cell cultures. Their intra- and extra-cellular environment is responsible for decreased sensor sensitivity due to interferences and the complete array of biological reactions, resulting in low sel ...
... Cell-based sensors have used living organisms such as bacteria, yeast, algae, fungi, animals and plants cell cultures. Their intra- and extra-cellular environment is responsible for decreased sensor sensitivity due to interferences and the complete array of biological reactions, resulting in low sel ...
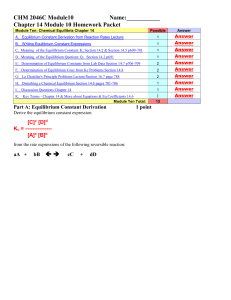
CHEM 1212 Module Ten-Chapter 16 Name
... __________________ 1. If all the reactants and the products are in the same phase (such as gases), then the we say the system is in _______________equilibrium. __________________ 2. If at least one of the reactants or one of the products is not in the same phase, then we say the system is in _______ ...
... __________________ 1. If all the reactants and the products are in the same phase (such as gases), then the we say the system is in _______________equilibrium. __________________ 2. If at least one of the reactants or one of the products is not in the same phase, then we say the system is in _______ ...
Ni recovery using KOH, NaOH, and NH4OH in the presence of
... During production of the platinum group metals (PGMs) in South Africa, base metals are leached first. A significant amount of iron is also co-leached, which in turn is removed by precipitation as ammonium jarosite. Substantial amounts of nickel have been found entrained in this precipitate, which is ...
... During production of the platinum group metals (PGMs) in South Africa, base metals are leached first. A significant amount of iron is also co-leached, which in turn is removed by precipitation as ammonium jarosite. Substantial amounts of nickel have been found entrained in this precipitate, which is ...
chemical reaction equation - parmod cobra insititution.
... The nature and identity of products totally changes from the reactants. Observations which determines whether the chemical reaction has taken place or not. (a) Chemical reaction must be associated with change in temperature i.e. Heat should be either evolved or absorbed. (b) The reaction must occur ...
... The nature and identity of products totally changes from the reactants. Observations which determines whether the chemical reaction has taken place or not. (a) Chemical reaction must be associated with change in temperature i.e. Heat should be either evolved or absorbed. (b) The reaction must occur ...
TEKS 8 - UNT College of Education
... Another way to remove ions from a solution is to combine an acid and a base. The two will neutralize forming water and a salt. One more way for the ions to be taken out of the water is for some of the ions to escape as a gas. Conclusion Chemical reactions are often accompanied by the appearance of g ...
... Another way to remove ions from a solution is to combine an acid and a base. The two will neutralize forming water and a salt. One more way for the ions to be taken out of the water is for some of the ions to escape as a gas. Conclusion Chemical reactions are often accompanied by the appearance of g ...
Mission Statement
... Online Reading and Assignments http://www.mahopac.k12.ny.us/mhs/teachers/langellam/ ...
... Online Reading and Assignments http://www.mahopac.k12.ny.us/mhs/teachers/langellam/ ...
Equation of state - Wikipedia, the free encyclopedia
... ideal gas law and does predict the formation of a liquid phase, the agreement with experimental data is limited for conditions where the liquid forms. While the van der Waals equation is commonly referenced in text-books and papers for historical reasons, it is now obsolete. Other modern equations o ...
... ideal gas law and does predict the formation of a liquid phase, the agreement with experimental data is limited for conditions where the liquid forms. While the van der Waals equation is commonly referenced in text-books and papers for historical reasons, it is now obsolete. Other modern equations o ...
Chapter 1
... are v x , v y , and v z . Its speed is v = (v x2 + v 2y + v z2 ) 2 , and its translational energy is εtr = 12 mv 2 , where m is the molecule’s mass. The x component of its momentum is mv x . When the molecule collides with a wall parallel to the y z plane, let us assume that the x component of its v ...
... are v x , v y , and v z . Its speed is v = (v x2 + v 2y + v z2 ) 2 , and its translational energy is εtr = 12 mv 2 , where m is the molecule’s mass. The x component of its momentum is mv x . When the molecule collides with a wall parallel to the y z plane, let us assume that the x component of its v ...
Formic acid oxidation reaction on a PdxNiy bimetallic nanoparticle
... stabilizer, and catalyst support for Pd. And the as-synthesized Pd/GR electrocatalysts showed increased electrochemical surface area and significantly enhanced catalytic activity for FAOR compared with the conventional Pd/C electrocatalysts in terms of peak current density and peak potential. Hong e ...
... stabilizer, and catalyst support for Pd. And the as-synthesized Pd/GR electrocatalysts showed increased electrochemical surface area and significantly enhanced catalytic activity for FAOR compared with the conventional Pd/C electrocatalysts in terms of peak current density and peak potential. Hong e ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.