Solution - ZOMUedu
... ■ 1 M = 1 mol solute/ liter solution ○ Dilution = adding more solvent to a known solution ■ The moles of solute stay the same ■ M1V1 = M2V2 ○ Stock solution = a solution of known concentration that is used to make more dilute solutions Precipitation ○ Precipitation = when aqueous solutions of ionic ...
... ■ 1 M = 1 mol solute/ liter solution ○ Dilution = adding more solvent to a known solution ■ The moles of solute stay the same ■ M1V1 = M2V2 ○ Stock solution = a solution of known concentration that is used to make more dilute solutions Precipitation ○ Precipitation = when aqueous solutions of ionic ...
Ch. 2 - Ltcconline.net
... B. Water’s polarity leads to H bonding C. Hydrogen bonds make liquid water cohesive 1. H bonds last for only a few trillionths of a second 2. cohesion and adhesion 3. surface tension D. Hydrogen bonds of water moderate temperature 1. heat 2. temperature E. Ice is less dense than water F. water is a ...
... B. Water’s polarity leads to H bonding C. Hydrogen bonds make liquid water cohesive 1. H bonds last for only a few trillionths of a second 2. cohesion and adhesion 3. surface tension D. Hydrogen bonds of water moderate temperature 1. heat 2. temperature E. Ice is less dense than water F. water is a ...
Solutions Intro
... the gold and copper that make up a gold ring or the mixture of copper and zinc that make up bronze are also solutions for the same reason. In a solution the major part is known as the solvent. So in air (78% nitrogen) nitrogen is the solvent. In most solutions, water is the solvent because water is ...
... the gold and copper that make up a gold ring or the mixture of copper and zinc that make up bronze are also solutions for the same reason. In a solution the major part is known as the solvent. So in air (78% nitrogen) nitrogen is the solvent. In most solutions, water is the solvent because water is ...
AP Chemistry Predicting Products Tutorial
... 1. A solution of sulfuric acid is added to a solution of barium hydroxide until the same number of moles of each compound has been added. 4H+ + SO42- + Ba2+ + 2OH- BaSO4 + 2H2O 2. A solution of sodium hydroxide is added to a solution of sodium dihydrogen phosphate until the same number of moles of ...
... 1. A solution of sulfuric acid is added to a solution of barium hydroxide until the same number of moles of each compound has been added. 4H+ + SO42- + Ba2+ + 2OH- BaSO4 + 2H2O 2. A solution of sodium hydroxide is added to a solution of sodium dihydrogen phosphate until the same number of moles of ...
II. Acids and Bases
... Therefore, the value of Kw is 1.0 x 10-14. This is also the product of [H+] and [OH-] for other solutions. 8. Because concentrations of H+ ions are often small numbers expressed in scientific notation, chemists adopted an easier way to express H+ ion concentration using a pH scale based on common lo ...
... Therefore, the value of Kw is 1.0 x 10-14. This is also the product of [H+] and [OH-] for other solutions. 8. Because concentrations of H+ ions are often small numbers expressed in scientific notation, chemists adopted an easier way to express H+ ion concentration using a pH scale based on common lo ...
Acids - IGChemistry
... Bases are often found in everyday products such as many cleaning products (sodium hydroxide), antacid products (magnesium hydroxide )and fertilisers (ammonia). It is a common misconception that bases are not as dangerous as acids. In fact, many bases can be as much or more corrosive than many acids. ...
... Bases are often found in everyday products such as many cleaning products (sodium hydroxide), antacid products (magnesium hydroxide )and fertilisers (ammonia). It is a common misconception that bases are not as dangerous as acids. In fact, many bases can be as much or more corrosive than many acids. ...
ELECTROCHEMISTRY / INTERFACIAL KINETICS
... Fe(s) + H2O ¾ Fe(OH)(surface) + H+(aq) + eFe(OH)(surface) ¾FeOH+(aq) + eFe(OH)+(aq) + H+(aq) ¾ Fe2+(aq)+H2O Use your value of b from part i) to deduce the rate determining step in this mechanism. iii) If the pH of the solution was increased by one unit, by what value would the current values alter? ...
... Fe(s) + H2O ¾ Fe(OH)(surface) + H+(aq) + eFe(OH)(surface) ¾FeOH+(aq) + eFe(OH)+(aq) + H+(aq) ¾ Fe2+(aq)+H2O Use your value of b from part i) to deduce the rate determining step in this mechanism. iii) If the pH of the solution was increased by one unit, by what value would the current values alter? ...
pH Scale and Concentration Date: Chemistry!
... It has a higher concentration of H 3O+ than OH– and causes litmus to turn blue. It has a higher concentration of OH – than H3O + and causes litmus to turn blue. It has a higher concentration of H 3O+ than OH– and causes methyl orange to turn yellow. It has a higher concentration of OH – than H 3O+ a ...
... It has a higher concentration of H 3O+ than OH– and causes litmus to turn blue. It has a higher concentration of OH – than H3O + and causes litmus to turn blue. It has a higher concentration of H 3O+ than OH– and causes methyl orange to turn yellow. It has a higher concentration of OH – than H 3O+ a ...
Intro to Soln Stoich
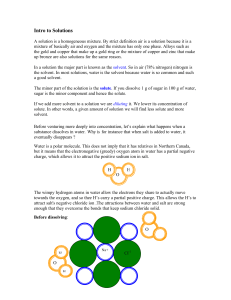
... Water is the “universal solvent” ◦ O-H bonds are covalent, e- not shared equally ◦ Oxygen has a slight negative, hydrogen slight positive Polar molecule Oxygen has a strong attraction to cations, hydrogen to anions ...
... Water is the “universal solvent” ◦ O-H bonds are covalent, e- not shared equally ◦ Oxygen has a slight negative, hydrogen slight positive Polar molecule Oxygen has a strong attraction to cations, hydrogen to anions ...
Working with solutions
... O A strong acid will produce more hydrogen ions than a weak one. O A strong base will produce more hydroxide ions than a weak one. ...
... O A strong acid will produce more hydrogen ions than a weak one. O A strong base will produce more hydroxide ions than a weak one. ...
Reactions between Solutions
... Date: ___/___/______ Block 1 2 3 4 5 6 7 8 Reactions between Solutions Experiment #16 ...
... Date: ___/___/______ Block 1 2 3 4 5 6 7 8 Reactions between Solutions Experiment #16 ...
Answer on Question #44399 – Chemistry – Other HC2O4 − + HOH
... Answer According to the Brønsted–Lowry theory, an acid is a species able to lose, or "donate" a proton (H+) while a base is a species with the ability to gain, or "accept," a proton. The hydrogen oxalate ion can gain a proton acting as a base towards water, while the latter donates proton acting as ...
... Answer According to the Brønsted–Lowry theory, an acid is a species able to lose, or "donate" a proton (H+) while a base is a species with the ability to gain, or "accept," a proton. The hydrogen oxalate ion can gain a proton acting as a base towards water, while the latter donates proton acting as ...
Acids and Bases The pH Scale
... aqueous solution. It is always associated with another water molecule in the form of H3O!. As indicated by the double arrows, this is a reversible reaction that reaches a state of dynamic equilibrium when water molecules dissociate at the same rate that they are being reformed from H! and OH". At th ...
... aqueous solution. It is always associated with another water molecule in the form of H3O!. As indicated by the double arrows, this is a reversible reaction that reaches a state of dynamic equilibrium when water molecules dissociate at the same rate that they are being reformed from H! and OH". At th ...
Challenge - ChemistryIBWYA
... particles, or ions, in the solution. [Charged particles are created when a substance either dissociates in or reacts with the solvent (usually water) to form ions.] The more ions the solution contains, the greater the amount of electrical current the solution can carry—in other words, the better a c ...
... particles, or ions, in the solution. [Charged particles are created when a substance either dissociates in or reacts with the solvent (usually water) to form ions.] The more ions the solution contains, the greater the amount of electrical current the solution can carry—in other words, the better a c ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.